2022
196. Chow, R.W., H. Fukui, W.X. Chan, K.S.J. Tan, S. Roth, A.L. Duchemin, N. Messaddeq, H. Nakajima, F. Liu, N. Faggianelli-Conrozier, A.S. Klymchenko, Y. Choon Hwai, N. Mochizuki, J. Vermot, Cardiac forces regulate zebrafish heart valve delamination by modulating Nfat signaling . PLoS Biology, 2022. 20(1):e3001505. doi: 10.1371/journal.pbio.3001505.
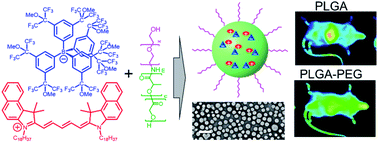
195. Sobska, J., B. Andreiuk, I.O. Aparin, A. Reisch, W. Krezel, A.S. Klymchenko, Counterion-insulated near-infrared dyes in biodegradable polymer nanoparticles for in vivo imaging . Nanoscale Advances, 2022. 4(1): p. 39-48. doi: 10.1039/d1na00649e.
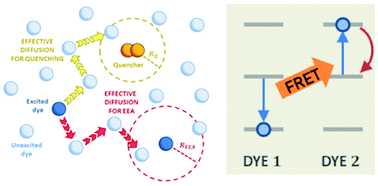
194. Fanciullo, G., I. Conti, P. Didier, A. Klymchenko, J. Léonard, M. Garavelli, I. Rivalta, Modelling quenching mechanisms of disordered molecular systems in the presence of molecular aggregates . Physical Chemistry Chemical Physics, 2022. 24(3): p. 1787-1794. doi: 10.1039/d1cp04260b.
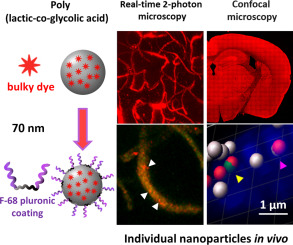
193. Khalin, I., C. Severi, D. Heimburger, A. Wehn, F. Hellal, A. Reisch, A.S. Klymchenko, N. Plesnila, Dynamic tracing using ultra-bright labeling and multi-photon microscopy identifies endothelial uptake of poloxamer 188 coated poly(lactic-co-glycolic acid) nano-carriers in vivo . Nanomedicine: Nanotechnology, Biology, and Medicine, 2022. 40: 102511.
doi: 10.1016/j.nano.2021.102511.
192. Combes, A., K.N. Tang, A.S. Klymchenko, A. Reisch, Protein-like particles through nanoprecipitation of mixtures of polymers of opposite charge . Journal of Colloid and Interface Science, 2022. 607(Pt 2): p. 1786-1795. doi: 10.1016/j.jcis.2021.09.080.
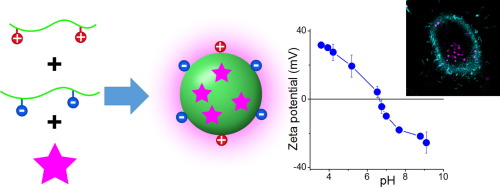
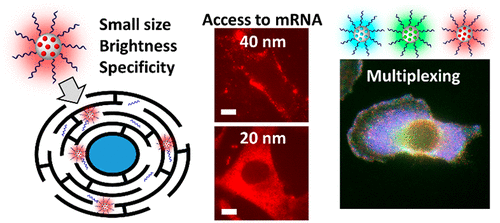
191. Egloff, S., N. Melnychuk, E. Cruz Da Silva, A, Reisch, S, Martin, A.S. Klymchenko, Amplified fluorescence in situ hybridization by small and bright dye-loaded polymeric nanoparticles . ACS Nano. 2022. 16(1): p. 1381-1394. doi: 10.1021/acsnano.1c09409.
2021
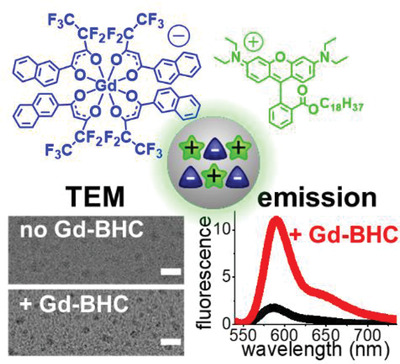
190. Severi, C., S. Lahtinen, J. Rosenberg, A. Reisch, T. Soukka, A.S. Klymchenko, Lanthanide-based bulky counterions against aggregation-caused quenching of dyes in fluorescent polymeric nanoparticles. Aggregate, Wiley, 2021, e130. doi: 10.1002/agt2.130.
189. Carravilla, P., A. Dasgupta, G. Zhurgenbayeva, D.I. Danylchuk, A.S. Klymchenko, E. Sezgin, C. Eggeling, Long-term STED imaging of membrane packing and dynamics by exchangeable polarity-sensitive dyes. Biophysics Reports, 2021. 1(2): 100023. doi: 10.1016/j.bpr.2021.100023.
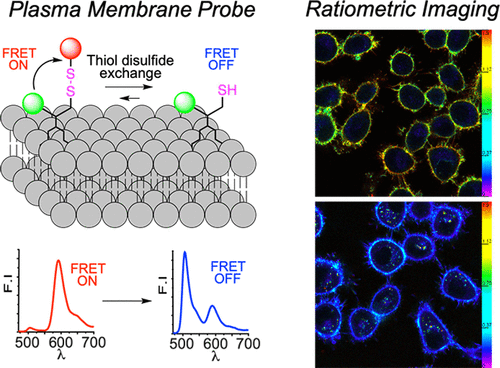
188. Mukherjee, T., S. Kanvah, A.S. Klymchenko, M. Collot, Probing variations of reduction activity at the plasma membrane using a targeted ratiometric FRET probe . ACS Applied Materials & Interfaces, 2021. 13(34): p. 40315-40324. doi: 10.1021/acsami.1c11069.
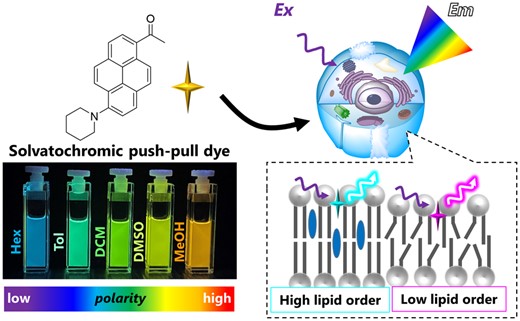
187. Niko, Y., A.S. Klymchenko, Emerging solvatochromic push-pull dyes for monitoring the lipid order of biomembranes in live cells . The Journal of Biochemistry, 2021. 170(2) : p. 163-174. doi: 10.1093/jb/mvab078.
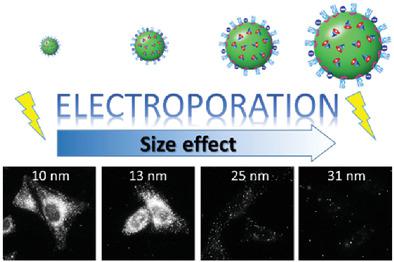
186.Egloff, S., A. Runser, A. Klymchenko, A. Reisch, Size-dependent electroporation of dye-loaded polymer nanoparticles for efficient and safe intracellular delivery. Small Methods, 2021. 5(2): 2000947. doi :10.1002/smtd.202000947.
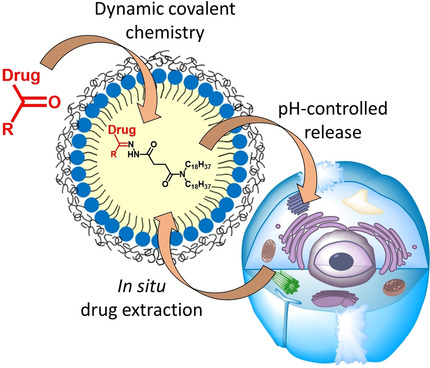
185. Liu, F., Y. Niko, R. Bouchaala, L. Mercier, O. Lefebvre, B. Andreiuk, T. Vandamme, J.G. Goetz, N. Anton, A. Klymchenko, Drug-Sponge Lipid Nanocarrier for in Situ Cargo Loading and Release Using Dynamic Covalent Chemistry. Angewandte Chemie-International Edition, 2021. 60(12): p. 6573-6580. doi: 10.1002/anie.202014259.
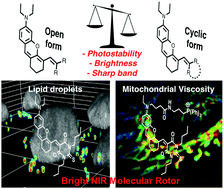
184. Mukherjee, T., R.J. Martinez-Sanchez, Fam, K.T., S. Bou, L. Richert, D. Garnier, Y. Mely, S. Kanvah, A.S. Klymchenko, M. Collot, Near infrared emitting molecular rotor based on merocyanine for probing the viscosity of cellular lipid environments. Materials Chemistry Frontiers, 2021. 5(5): p. 2459-2469. doi: 10.1039/d0qm00872a.
183. Sot, J., I. Esnal, B.G. Monasterio, R. León-Irra, Y. Niko, F.M. Goñi, A. Klymchenko A, A. Alonso, Phase-selective staining of model and cell membranes, lipid droplets and lipoproteins with fluorescent solvatochromic pyrene probes. Biochimica et Biophysica Acta - Biomembranes, 2021. 1863(1):183470. doi: 10.1016/j.bbamem.2020.183470.
182. Klymchenko, A.S., F. Liu, M. Collot, N. Anton, Dye-Loaded Nanoemulsions: Biomimetic Fluorescent Nanocarriers for Bioimaging and Nanomedicine. Advanced Healthcare Materials, 2021. 10(1):e2001289. doi: 10.1002/adhm.202001289.
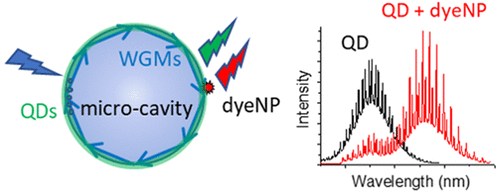
181. Jana, S., X. Xu, A. Klymchenko, A. Reisch, T. Pons, Microcavity-enhanced fluorescence energy transfer from Quantum Dot excited whispering gallery modes to acceptor dye. ACS Nano, 2021. 15(1): p. 1445-1453. doi: 10.1021/acsnano.0c08772.
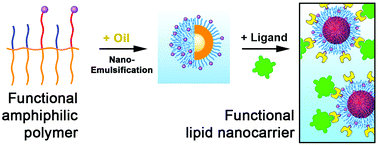
180. Rehman, A.U., N. Anton, S. Bou, J. Schild, N. Messaddeq, T. Vandamme, S. Akram, A. Klymchenko, M. Collot, Tunable functionalization of nano-emulsions using amphiphilic polymers. Soft Matter, 2021. 17(7): p. 1788-1795. doi: 10.1039/d0sm01952f.
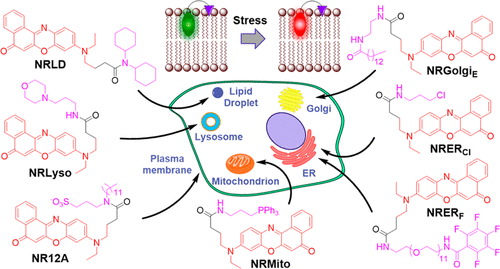
179. Danylchuk, D.I., P.H. Jouard, A.S. Klymchenko, Targeted solvatochromic fluorescent probes for imaging lipid order in organelles under oxidative and mechanical stress. Journal of the American Chemical Society, 2021. 143(2): p. 912-924. doi: 10.1021/jacs.0c10972.
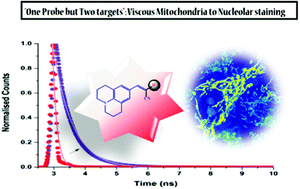
178. Mukherjee, T., V. Soppina, R. Ludovic, Y. Mély, A.S. Klymchenko, M. Collot, S. Kanvah, Live-cell imaging of the nucleolus and mapping mitochondrial viscosity with a dual function fluorescent probe. Organic & Biomolecular Chemistry, 2021. 19(15): p. 3389-3395. doi: 10.1039/d0ob02378g.
177. Ashokkumar, P., M. Collot, A.S. Klymchenko, Fluorogenic squaraine dendrimers for background-free imaging of integrin receptors in cancer cells. Chemistry: A European Journal, 2021. 27(22): p. 6795-6803. doi: 10.1002/chem.202100480.
176. Egloff, S., N. Melnychuk, A. Reisch, S. Martin, A.S. Klymchenko, Enzyme-free amplified detection of cellular microRNA by light-harvesting fluorescent nanoparticle probes. Biosensors and Bioelectronics, 2021. 179:113084. doi: 10.1016/j.bios.2021.113084.
175. Barberio, M., M. Al-Taher, E. Felli, A.H. Ashoka, J. Marescaux, A. Klymchenko, M. Diana, Intraoperative ureter identification with a novel fluorescent catheter. Scientific Reports, 2021. 11(1): 4501. doi: 10.1038/s41598-021-84121-z.
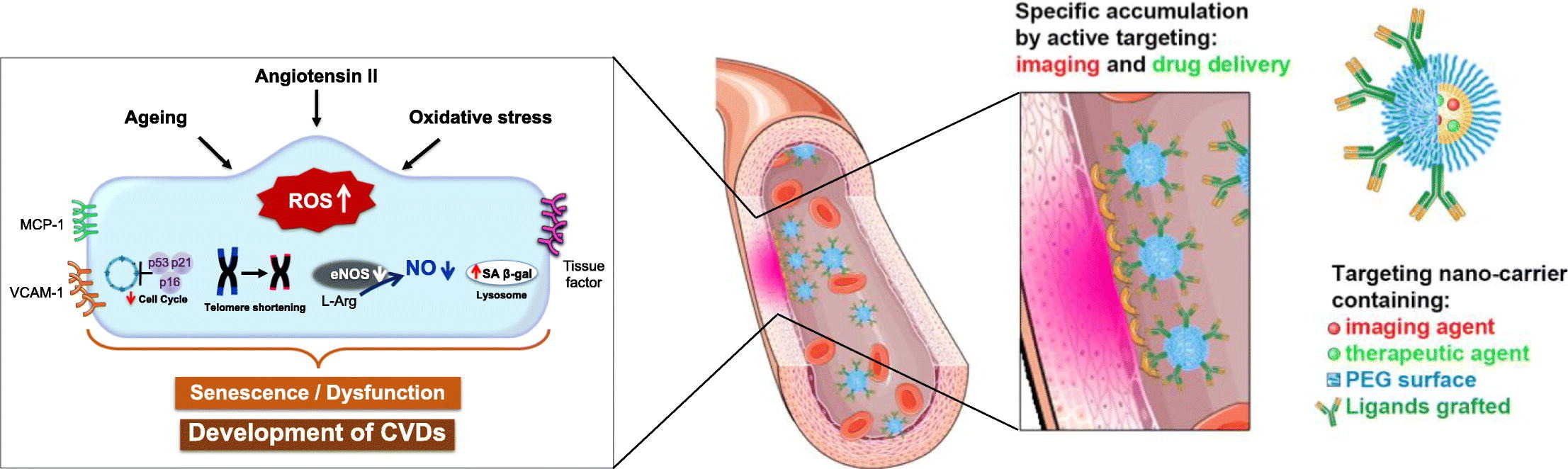
174. Belcastro, E., A.U. Rehman, L. Remila, S.H. Park, D.S. Gong, N. Anton, C. Auger, O. Lefebvre, J.G. Goetz, M. Collot, A.S. Klymchenko, T.F. Vandamme, V.B. Schini-Kerth, Fluorescent nanocarriers targeting VCAM-1 for early detection of senescent endothelial cells. Nanomedicine, 2021. 34:102379. doi: 10.1016/j.nano.2021.102379.
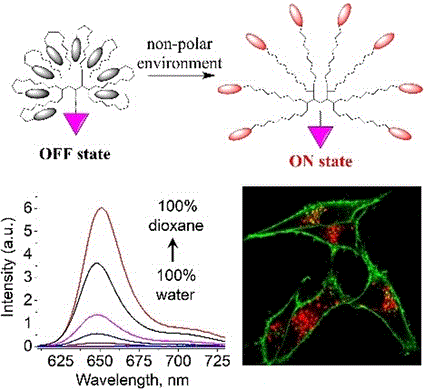
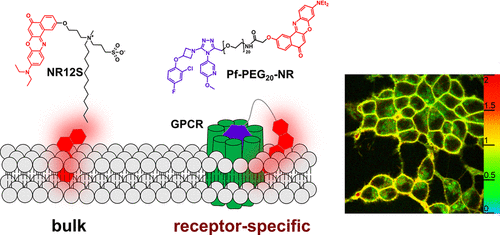
173. Hanser, F., C. Marsol, C. Valencia, P. Villa, A.S. Klymchenko, D. Bonnet, J. Karpenko, Nile Red-based GPCR ligands as ultrasensitive probes of the local lipid microenvironment of the receptor. ACS Chemical Biology, 2021. 16(4): p. 651-660. doi: 10.1021/acschembio.0c00897.
172. Wang, X., S. Bou, A.S. Klymchenko, N. Anton, M. Collot, Ultrabright Green-emitting nanoemulsions based on natural lipids-BODIPY conjugates. Nanomaterials (Basel), 2021. 11(3): p. 826. doi: 10.3390/nano11030826.
171. Cubi, R., F. Bouhedda, M. Collot, A.S. Klymchenko, M. Ryckelynck, µIVC-Useq: a microfluidic-assisted high-throughput functionnal screening in tandem with next generation sequencing and artificial neural network to rapidly characterize RNA molecules.RNA, 2021. 27(7): p. 841-53. doi: 10.1261/rna.077586.120.
170. Fam, K.T., L. Saladin, A.S. Klymchenko, M. Collot, Confronting molecular rotors and self-quenched dimers as fluorogenic BODIPY systems to probe biotin receptors in cancer cells. Chemical Communications (Camb). 2021 57(39): p. 4807-4810. doi: 10.1039/d1cc00108f.
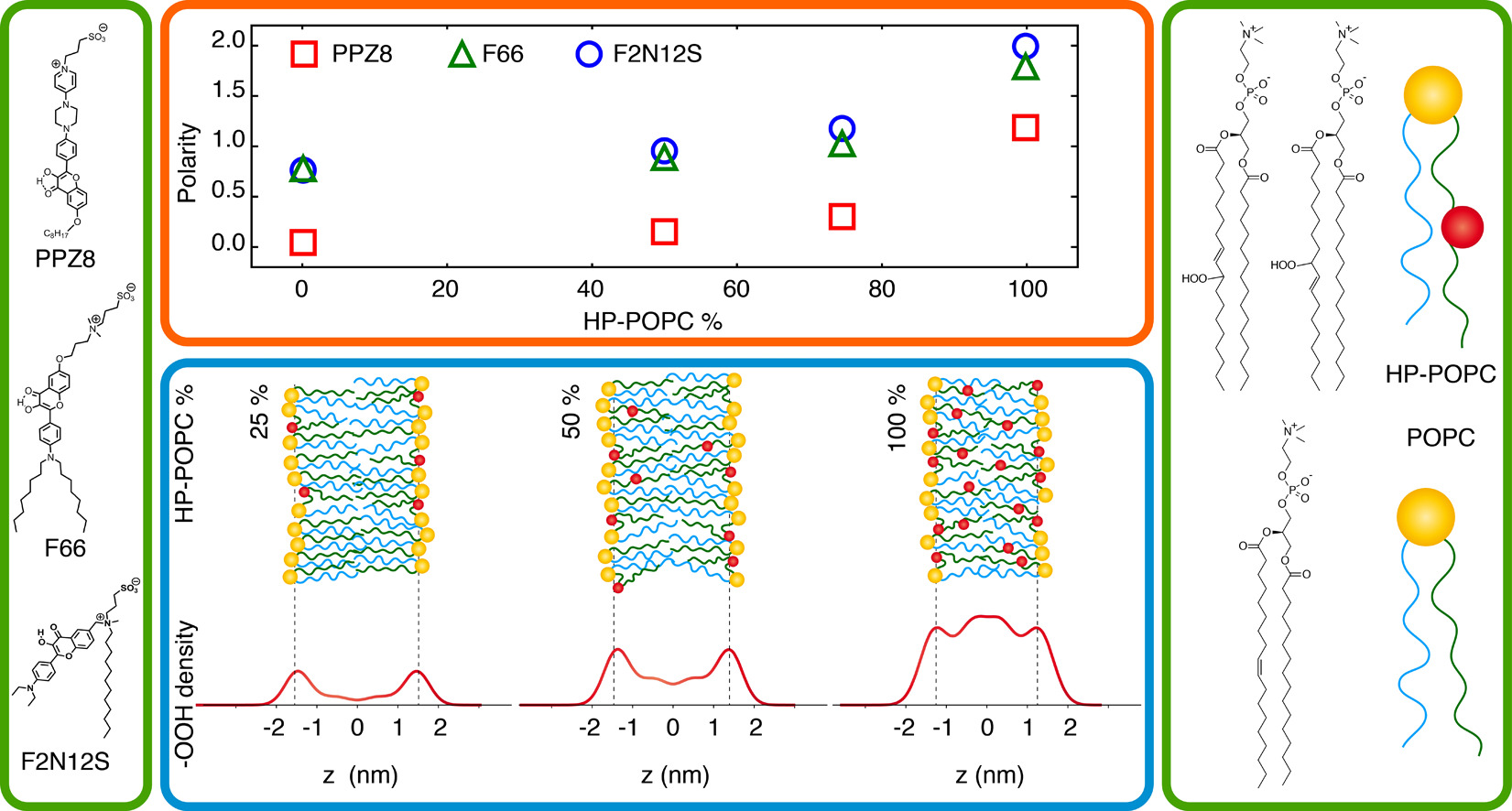
169. Junqueira, H., A.P. Schroder, F. Thalmann, A. Klymchenko, Y. Mély, M.S. Baptista, C.M. Marques, Molecular organization in hydroperoxidized POPC bilayers. Biochimica et Biophysica Acta – Biomembranes, 2021. 1863(10):183659. doi: 10.1016/j.bbamem.2021.183659.
168. Ashoka, A.H., A.S. Klymchenko, Ultrabright fluorescent polymeric nanofibers and coatings based on ionic dye insulation with bulky counterions. ACS Applied Materials & Interfaces, 2021. 13(24): p. 28889-28898. doi: 10.1021/acsami.1c06436.
167. Bou, S., A.S. Klymchenko, M. Collot, Fluorescent labeling of biocompatible block copolymers: synthetic strategies and applications in bioimaging. Materials Advances, 2021. 2(10): p. 3213-3233. doi: 10.1039/d1ma00110h.
166. Andreiuk, B., I. Aparin, A. Reisch, A.S. Klymchenko, Bulky Barbiturates as Non-toxic Ionic Dye Insulators for Enhanced Emission in Polymeric Nanoparticles. Chemistry: A European Journal, 2021. doi: 10.1002/chem.202101986.
165. Al-Taher, M., M. Barberio, E. Felli, V. Agnus, A.H. Ashoka, S. Gioux, A. Klymchenko, N. Bouvy, L. Stassen, J. Marescaux, M. Diana, Simultaneous multipurpose fluorescence imaging with IRDye® 800BK during laparoscopic surgery. Surgical Endoscopy 2021. 35(8): p. 4840-4848. doi: 10.1007/s00464-020-07931-8.
2020
164. Aparin, I.O., N. Melnychuk, A. Klymchenko, Ionic aggregation‐induced emission: bulky hydrophobic counterions light up dyes in polymeric nanoparticles. Advanced Optical Materials, 2020. 8(14): 2070058. doi10.1002/adom.202070058.
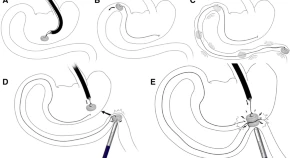
163. Watanabe, R., M. Barberio, S. Kanaji, A. Lapergola, A.H. Ashoka, B. Andreiuk, L. Guerriero, M. Pizzicannella, B. Seeliger, Y. Saida, H. Kaneko, M. Worreth, A. Saadi, J. Marescaux, A.S. Klymchenko, M. Diana, Hybrid fluorescent magnetic gastrojejunostomy: an experimental feasibility study in the porcine model and human cadaver. Surgical Endoscopy, 2020.34(3): p. 1393-1400. doi: 10.1007/s00464-019-06963-z.
162. Bouhedda, F., K.T. Fam, M. Collot, A. Autour, S. Marzi, A. Klymchenko, M. Ryckelynck, A dimerization-based fluorogenic dye-aptamer module for RNA imaging in live cells. Nature Chemical Biology, 2020. 16(1): p. 69-76. doi: 10.1038/s41589-019-0381-8.
161. Seeliger, B., V. Agnus, P. Mascagni, M. Barberio, F. Longo, A. Lapergola, D. Mutter, A.S. Klymchenko, M. Chand, J. Marescaux, M. Diana, Simultaneous computer-assisted assessment of mucosal and serosal perfusion in a model of segmental colonic ischemia. Surgical Endoscopy, 2020. 34(11): p. 4818-4827. doi: 10.1007/s00464-019-07258-z.
160. Runser, A., D. Dujardin, P. Ernst, A.S. Klymchenko, A. Reisch, Zwitterionic stealth dye-loaded polymer nanoparticles for intracellular imaging. ACS Applied Materials & Interfaces, 2020. 12(1): p. 117-125. doi: 10.1021/acsami.9b15396.
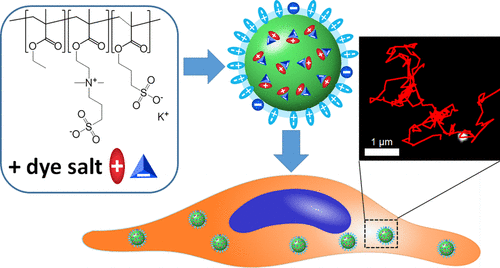
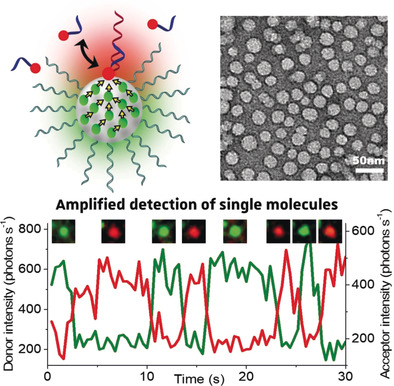
159. Melnychuk, N., S. Egloff, A. Runser, A. Reisch, A.S. Klymchenko, Light-harvesting nanoparticle probes for FRET-based detection of oligonucleotides with single-molecule sensitivity. Angewandte Chemie- International Edition, 2020. 59(17): p. 6811-6818. doi: 10.1002/anie.201913804.
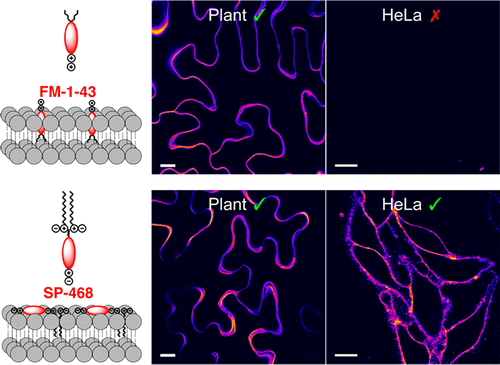
158. Collot, M., E. Boutant, K.T. Fam, L. Danglot, A.S. Klymchenko, Molecular tuning of styryl dyes leads to versatile and efficient plasma membrane probes for cell and tissue imaging. Bioconjugate Chemistry, 2020. 31(3): p. 875-883. doi: 10.1021/acs.bioconjchem.0c00023.
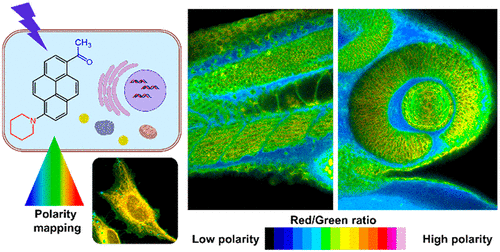
157. Valanciunaite, J., E. Kempf, H. Seki, D.I. Danylchuk, N. Peyriéras, Y. Niko, A.S. Klymchenko, Polarity mapping of cells and embryos by improved fluorescent solvatochromic pyrene probe. Analytical Chemistry, 2020. 92(9): p. 6512-6520. doi: 10.1021/acs.analchem.0c00023.
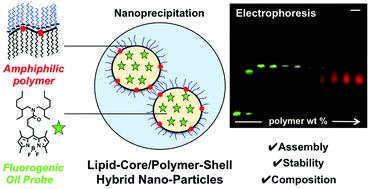
156. Bou, S., X. Wang, N. Anton, R. Bouchaala, A.S. Klymchenko, M. Collot, Lipid-core/polymer-shell hybrid nanoparticles: synthesis and characterization by fluorescence labeling and electrophoresis. Soft Matter, 2020. 16(17): p. 4173-4181. doi: 10.1039/d0sm00077a.
155. Barberio, M., M. Al-Taher, A. Forgione, A. Hoskere Ashoka, E. Felli, V. Agnus, J. Marescaux, A. Klymchenko, M. Diana, A novel method for near-infrared fluorescence imaging of the urethra during perineal and transanal surgery: demonstration in a cadaveric model. Colorectal Disease, 2020. 22(11): p. 1749-1753. doi: 10.1111/codi.15156.
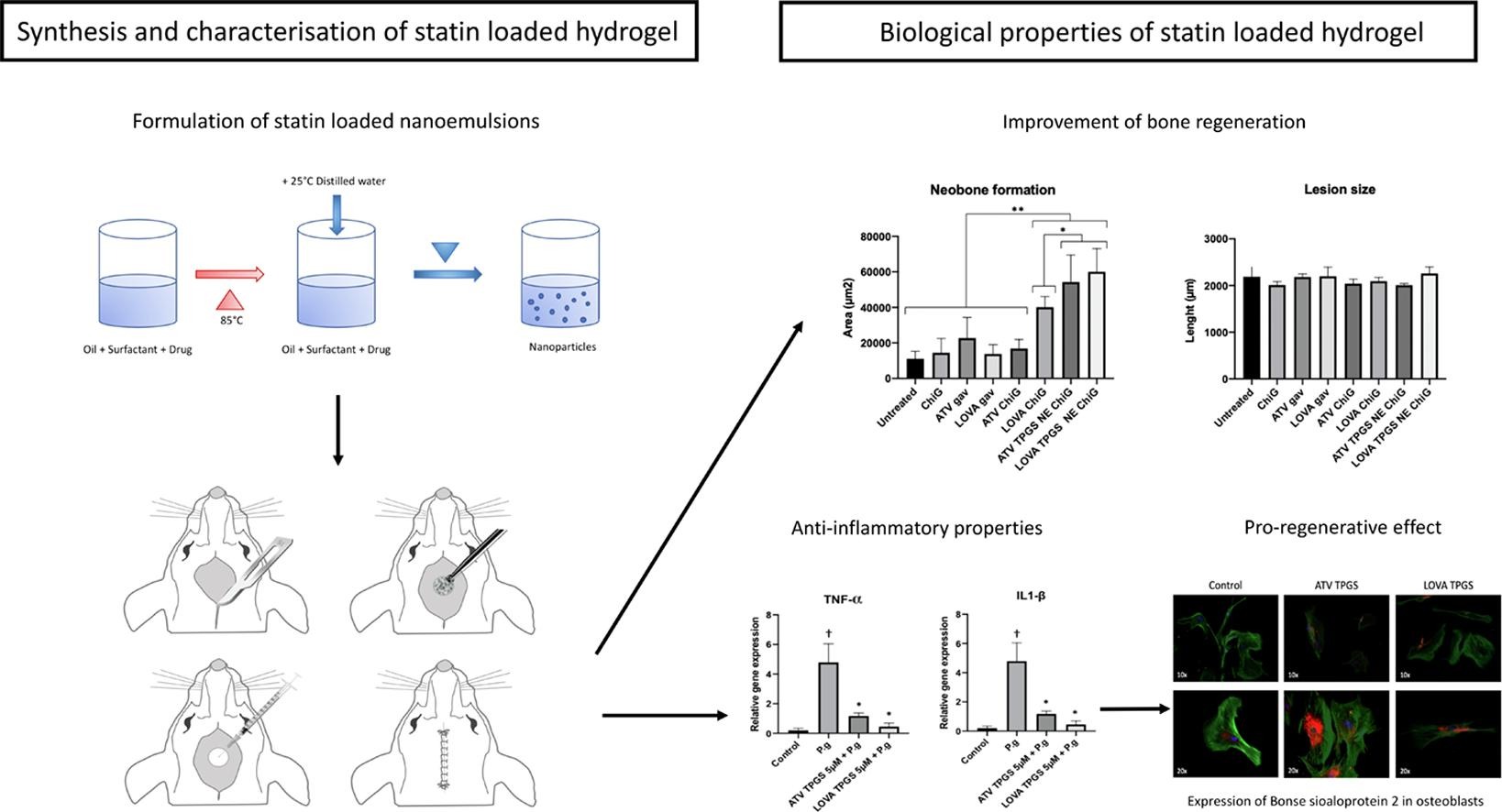
154. Petit, C., F. Batool, C. Stutz, N. Anton, A. Klymchenko, T. Vandamme, N. Benkirane-Jessel, O. Huck, Development of a thermosensitive statin loaded chitosan-based hydrogel promoting bone healing. International Journal of Pharmaceutics, 2020. 586:119534. doi: 10.1016/j.ijpharm.2020.119534.
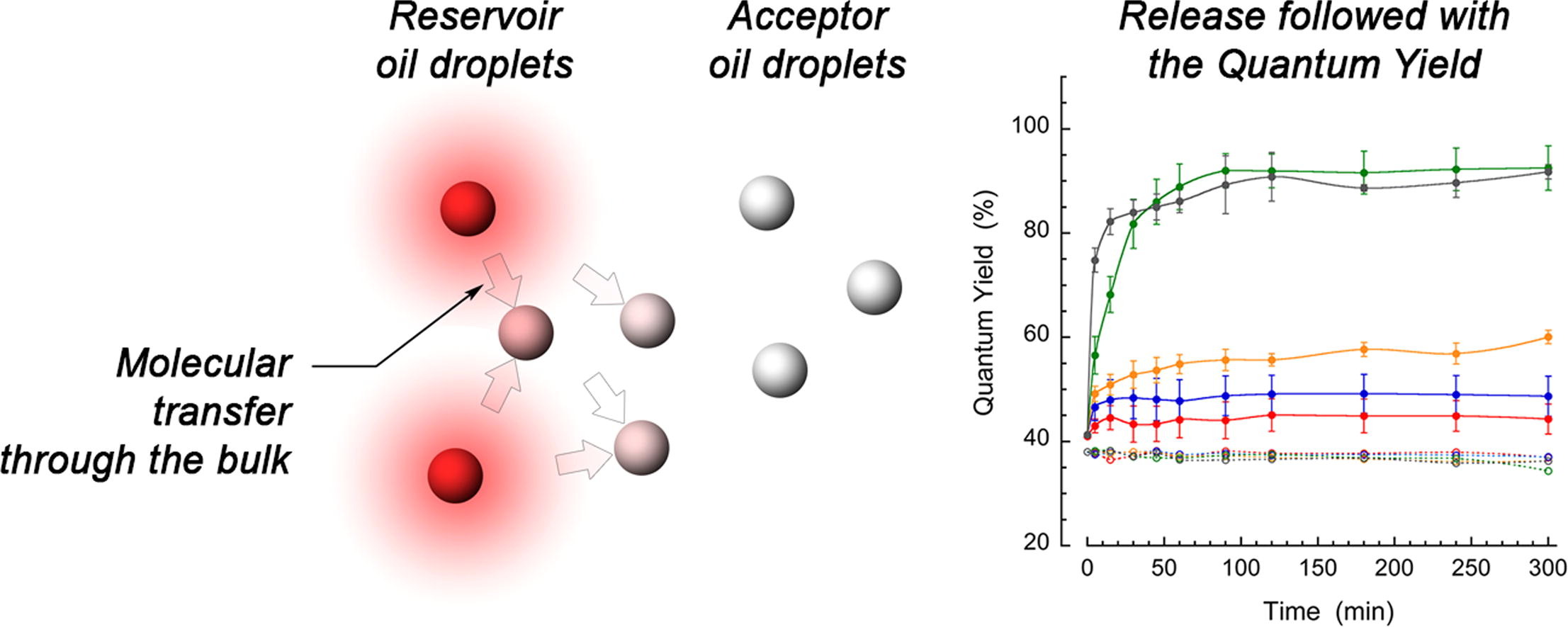
153. Wang, X., M. Collot, Z. Omran, T.F. Vandamme, A. Klymchenko, N. Anton, Further insights into release mechanisms from nano-emulsions, assessed by a simple fluorescence-based method. Journal of Colloid and Interface Science, 2020. 578: p. 768-778. doi: 10.1016/j.jcis.2020.06.028.
152. Ashokkumar, P., N. Adarsh, A.S. Klymchenko, Ratiometric nanoparticle probe based on FRET-amplified phosphorescence for oxygen sensing with minimal phototoxicity. Small, 2020. 16(32):e2002494. doi: 10.1002/smll.202002494.
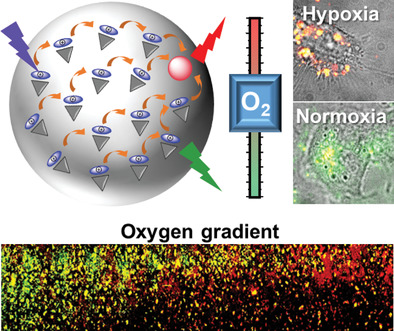
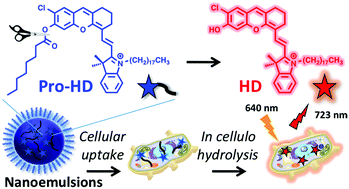
151. Bou, S., X. Wang, N. Anton, A.S. Klymchenko, M. Collot, Near infrared fluorogenic probe as a prodrug model for evaluating cargo release by nanoemulsions. Journal of Materials Chemistry B, 2020. 8(27): p. 5938-5944. doi: 10.1039/d0tb00783h.
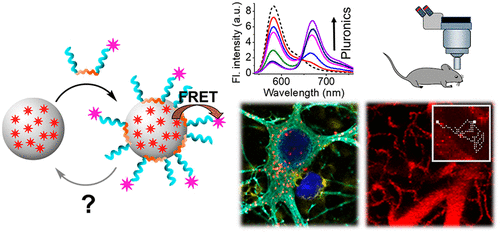
150. Khalin, I., D. Heimburger, N. Melnychuk, M. Collot, B. Groschup, F. Hellal, A. Reisch, N. Plesnila, A.S. Klymchenko, Ultrabright fluorescent polymeric nanoparticles with a stealth pluronic shell for live tracking in the mouse brain. ACS Nano, 2020. 14(8): p. 9755-9770. doi: 10.1021/acsnano.0c01505.
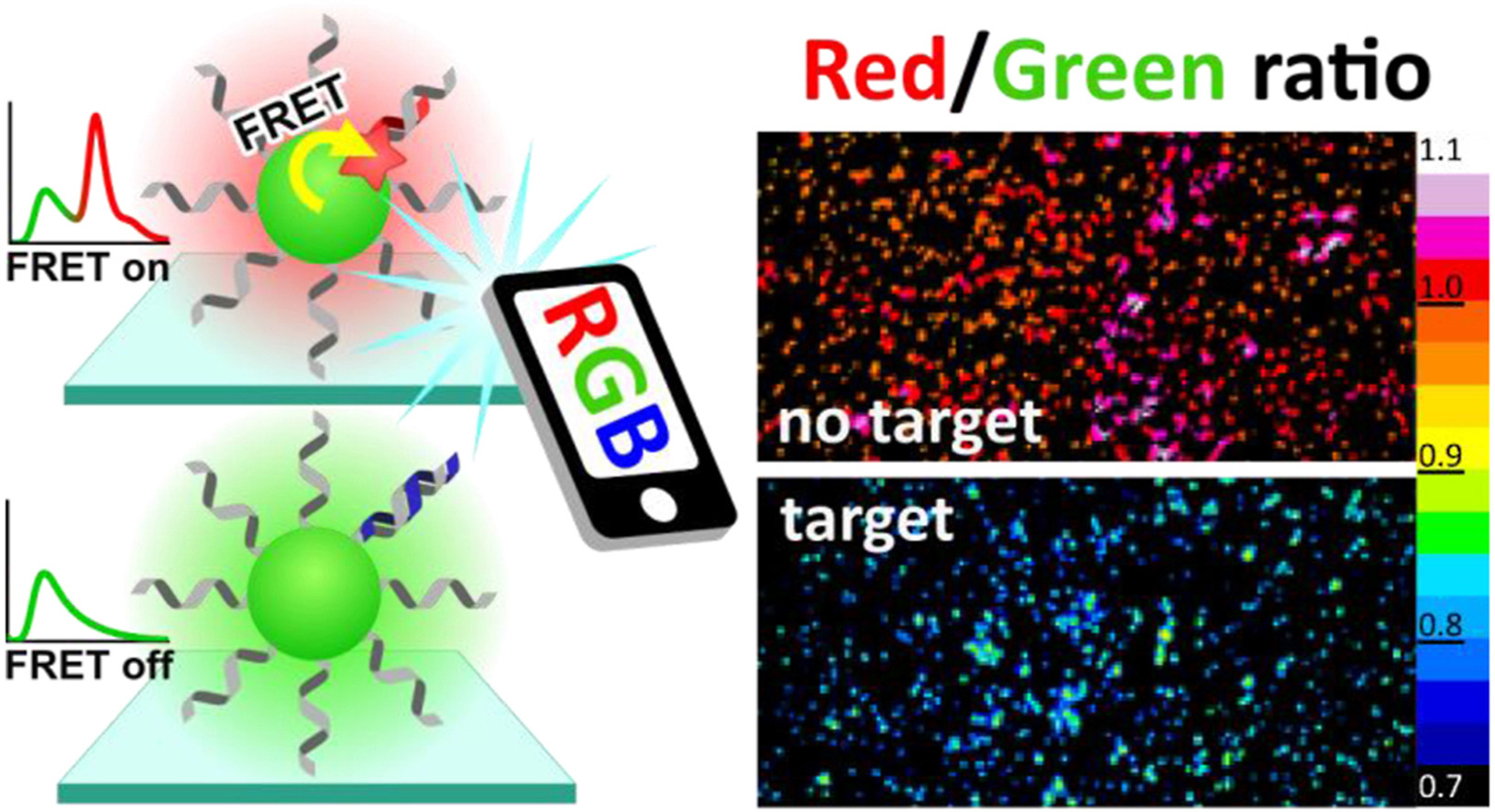
149. Severi, C., N. Melnychuk, A.S. Klymchenko,Smartphone-assisted detection of nucleic acids by light-harvesting FRET-based nanoprobe. Biosensors and Bioelectronics, 2020. 168:112515. doi: 10.1016/j.bios.2020.112515.
148. Ashoka, A.H., S.H. Kong, B. Seeliger, B. Andreiuk, R.V. Soares, M. Barberio, M. Diana, A.S. Klymchenko, Near-infrared fluorescent coatings of medical devices for image-guided surgery. Biomaterials, 2020. 261: 120306. doi: 10.1016/j.biomaterials.2020.120306.
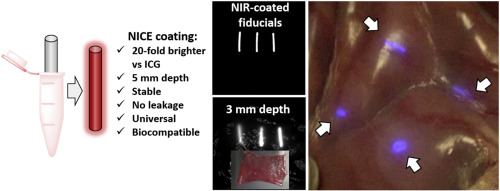
147. Barberio, M., M. Pizzicannella, A. Spota, A.H. Ashoka, V. Agnus, M. Al Taher, B. Jansen-Winkeln, I. Gockel, J. Marescaux, L. Swanström, S.H. Kong, E. Felli, A. Klymchenko, M. Diana, Preoperative endoscopic marking of the gastrointestinal tract using fluorescence imaging: submucosal indocyanine green tattooing versus a novel fluorescent over-the-scope clip in a survival experimental study. Surgical Endoscopy 2020, Sep 28. doi: 10.1007/s00464-020-07999-2.
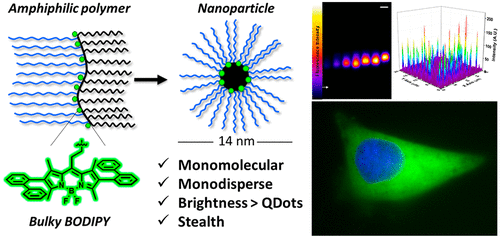
146. Collot, M., J. Schild, F.T. Fam, R. Bouchaala, A.S. Klymchenko, Stealth and bright monomolecular fluorescent organic nanoparticles based on folded amphiphilic polymer. ACS Nano, 2020. 14(10): p. 13924-13937. doi: 10.1021/acsnano.0c06348.
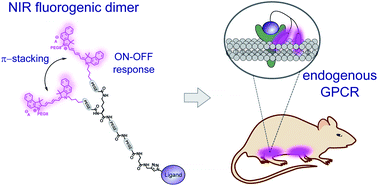
145. Esteoulle, L., F. Daubeuf, M. Collot, S. Riché, T. Durroux, D. Brasse, P. Marchand, I.A. Karpenko, A.S. Klymchenko, D. Bonnet, A near-infrared fluorogenic dimer enables background-free imaging of endogenous GPCRs in living mice. Chemical Science, 2020. 11(26): p. 6824-6829. doi: 10.1039/d0sc01018a.
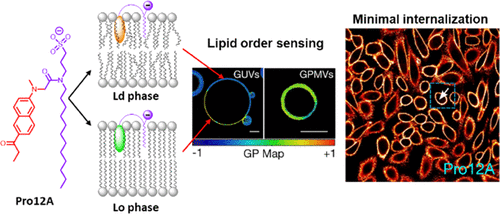
144. Danylchuk, D.I., E. Sezgin, P. Chabert, A.S. Klymchenko,Redesigning solvatochromic probe Laurdan for imaging lipid order selectively in cell plasma membranes. Analytical Chemistry, 2020. 92(21): p. 14798-14805. doi: 10.1021/acs.analchem.0c03559.
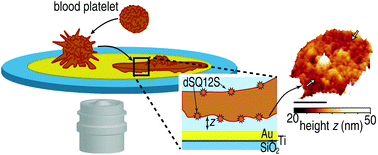
143. Zelená, A., S. Isbaner, D. Ruhlandt, A. Chizhik, C. Cassini, A.S. Klymchenko, J. Enderlein, A. Chizhik, S. Köster, Time-resolved MIET measurements of blood platelet spreading and adhesion. Nanoscale, 2020. 12(41): p. 21306-21315. doi: 10.1039/d0nr05611a.
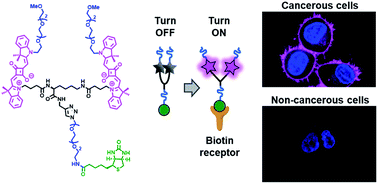
142. Fam, K.T., M. Collot, A.S. Klymchenko, Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chemical Science, 2020. 11(31): p. 8240-8248. doi: 10.1039/d0sc01973a.
2019
141. Jiang, M., J. Hu, F.K.H. White, J. Williamson, A.S. Klymchenko, A. Murthy, S.W. Workman, G.N. Tseng, S-Palmitoylation of junctophilin-2 is critical for its role in tethering the sarcoplasmic reticulum to the plasma membrane. Journal of Biological Chemistry, 2019. 294(36): p. 13487-13501. doi: 10.1074/jbc.RA118.006772.

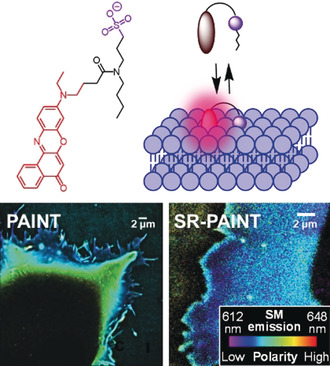
140. Danylchuk, D.I., S, Moon, K, Xu, A.S. Klymchenko, Switchable Solvatochromic Probes for Live-Cell Super-resolution Imaging of Plasma Membrane Organization. Angewandte Chemie- International Edition, 2019. 58(42): p. 14920-14924. doi: 10.1002/anie.201907690.
139. Buenaventura, T., S. Bitsi, W.E. Laughlin, T. Burgoyne, Z. Lyu, A.I. Oqua, H. Norman, E.R. McGlone, A.S. Klymchenko, I.R. Jr. Corrêa, A. Walker, A. Inoue, A. Hanyaloglu, J. Grimes, Z. Koszegi, D. Calebiro, G.A. Rutter, S.R. Bloom, B. Jones, A. Tomas, Agonist-induced membrane nanodomain clustering drives GLP-1 receptor responses in pancreatic beta cells.. PLoS Biology, 2019. 17(8): e3000097. doi: 10.1371/journal.pbio.3000097.
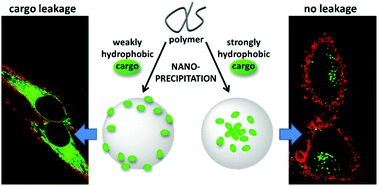
138. Trofymchuk, K., J. Valanciunaite, B. Andreiuk, A. Reisch, M. Collot, A. S. Klymchenko, BODIPY-loaded polymer nanoparticles: chemical structure of cargo defines leakage from nanocarrier in living cells. Journal of Materials Chemistry B, 2019. 7(34): p. 5199-5210.
137. Adarsh, N., A. S. Klymchenko, Ionic aggregation-induced emission dye with bulky counterions for preparation of bright near-infrared polymeric nanoparticles. Nanoscale, 2019. 11(29): p. 13977-13987.
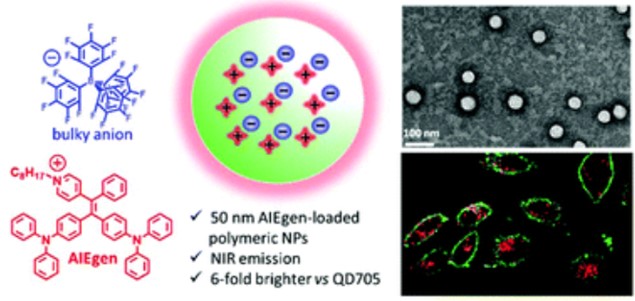
136. Ashokkumar P., A. H. Ashoka, M. Collot, A. Das, A. S. Klymchenko, A fluorogenic BODIPY molecular rotor as an apoptosis marker. Chemical Communications, 2019. 55(48): p. 6902-6905.
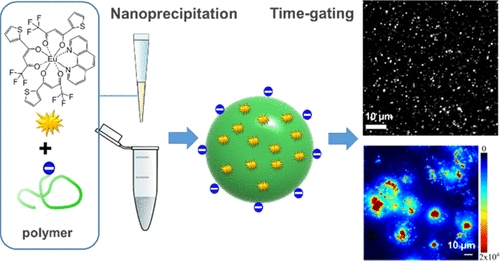
135. Dos Santos, M. C., A. Runser, H. Bartenlian, A. M. Nonat, L. J. Charbonnière, A. S. Klymchenko, N. Hildebrandt, A. Reisch, Lanthanide-Complex-Loaded Polymer Nanoparticles for Background-Free Single-Particle and Live-Cell Imaging. Chemistry of Materials, 2019. 31(11): p. 4034-4041.
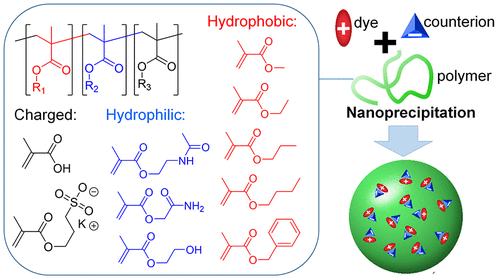
134. Rosiuk, V., A. Runser, A. Klymchenko, A. Reisch, Controlling Size and Fluorescence of Dye-Loaded Polymer Nanoparticles through Polymer Design. Langmuir, 2019. 35(21): p. 7009-7017.
133. Ashoka, A. H., P. Ashokkumar, Y. P. Kovtun, A. S. Klymchenko, Solvatochromic Near-Infrared Probe for Polarity Mapping of Biomembranes and Lipid Droplets in Cells under Stress. Journal of Physical Chemistry Letters, 2019. 10(10): p. 2414-2421.
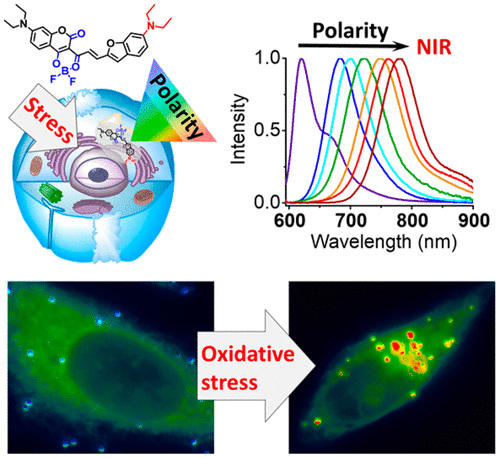
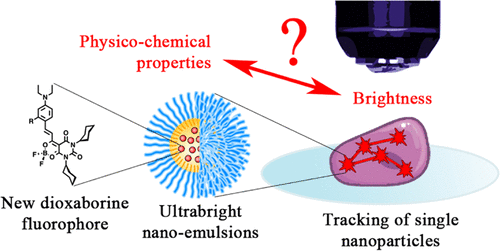
132. Wang, X., N. Anton, P. Ashokkumar, H. Anton, T. K. Fam, T. Vandamme, A. S. Klymchenko, M. Collot, Optimizing the Fluorescence Properties of Nanoemulsions for Single Particle Tracking in Live Cells. ACS Applied Materials & Interfaces, 2019. 11(14): p. 13079-13090.
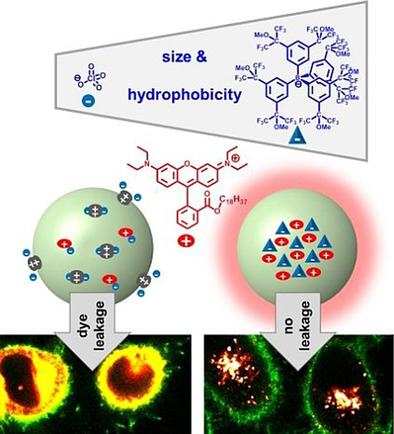
131. Andreiuk, B., A. Reisch, E. Bernhardt, A. S. Klymchenko, Fighting Aggregation Caused Quenching and Leakage of Dyes in Fluorescent Polymer Nanoparticles: Universal Role of Counterion. Chemistry - An Asian Journal, 2019. 14(6): p. 836-846.
130. Hyenne, V., S. Ghoroghi, M. Collot, S. Harlepp, J. Bauer, L. Mercier, I. Busnelli, O. Lefebvre, N. Fekonja, P. Machado, J. Bons, F. Delalande, A. I. Amor, S. G. Silva, F. J. Verweij, G. Van Niel, Y. Schwab, H. Peinado, C. Carapito, A. S. Klymchenko, J. G. Goetz, Studying the fate of tumor extracellular vesicles at high spatio-temporal resolution using the zebrafish embryo. Developmental Cell, 2019. 48(4): p. 554-572.e7.
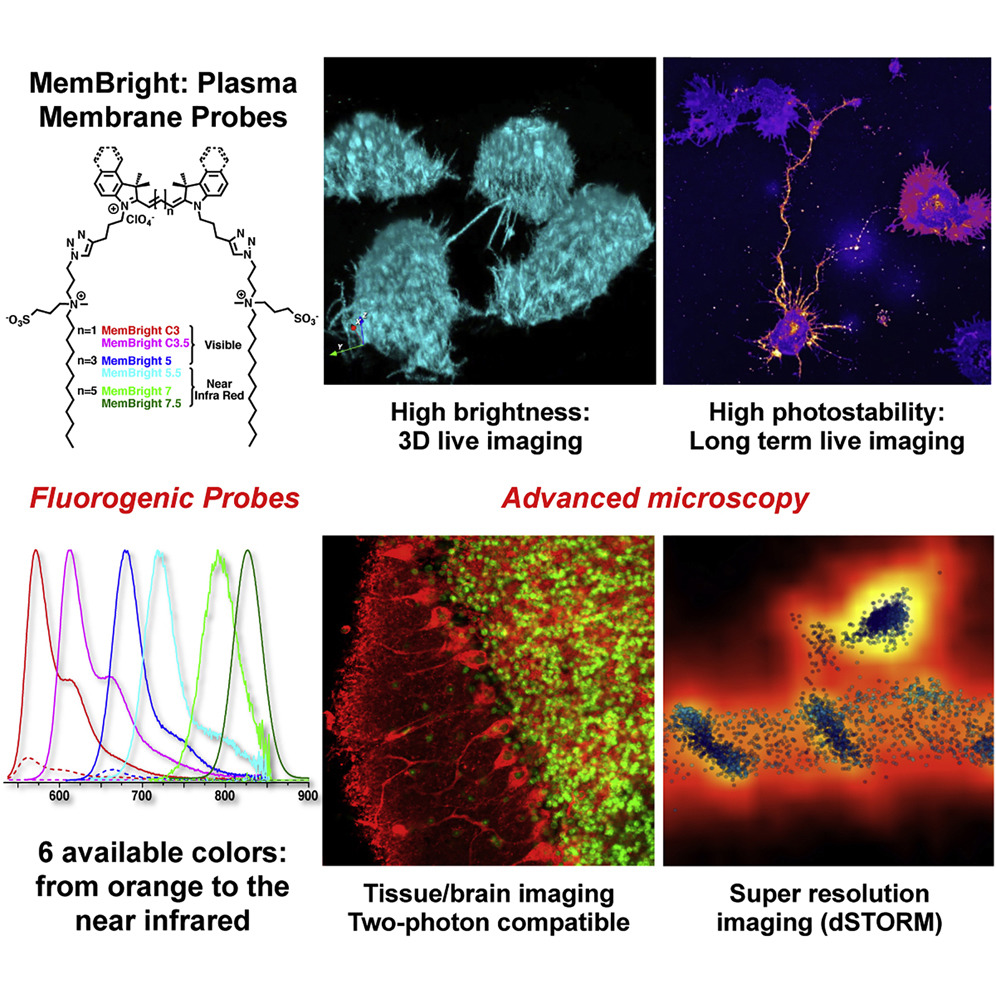
129. Collot, M., P. Ashokkumar, H. Anton, E. Boutant, O. Faklaris, T. Galli, Y. Mely, L. Danglot, and A. S. Klymchenko, MemBright: A Family of Fluorescent Membrane Probes for Advanced Cellular Imaging and Neuroscience. Cell Chem. Biol., 2019. 26(4): p.600-614.
128. Collot, M., E. Boutant, M. Lehmann and A.S. Klymchenko, BODIPY with Tuned Amphiphilicity as a Fluorogenic Plasma Membrane Probe. Bioconjugate Chemistry, 2019. 30(1): p. 192-199.
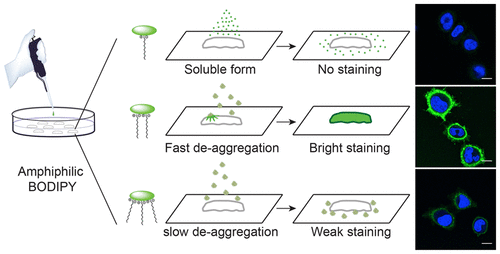
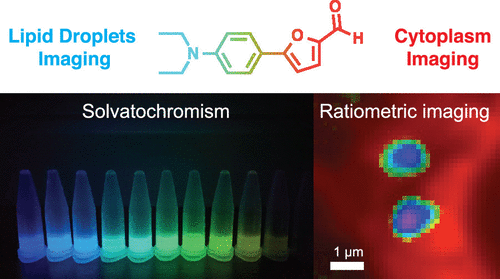
127. Collot, M., S. Bou, T.K. Fam, L. Richert, Y. Mely, L. Danglot and A.S. Klymchenko, Probing Polarity and Heterogeneity of Lipid Droplets in Live Cells Using a Push-Pull Fluorophore. Analytical Chemistry, 2019. 91(3): p. 1928-1935.
2018
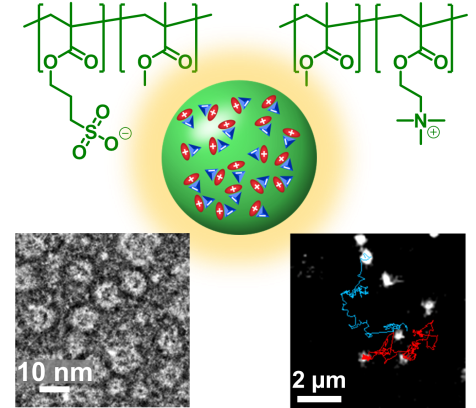
126. Reisch, A., D. Heimburger, P. Ernst, A. Runser, P. Didier, D. Dujardin, and A. S. Klymchenko, Protein‐Sized Dye‐Loaded Polymer Nanoparticles for Free Particle Diffusion in Cytosol. Advanced Functional Materials, 2018. 28(48): p. 1805157.
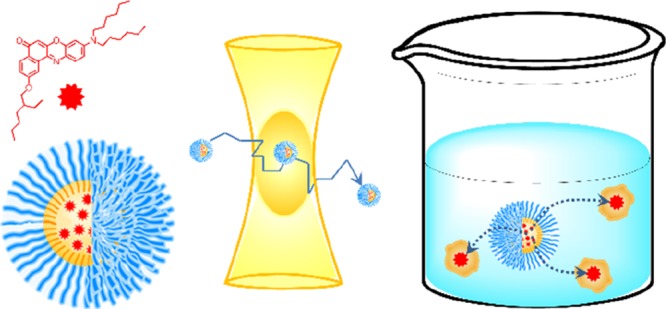
125. Bouchaala, R., L. Richert, N. Anton, T.F. Vandamme, S. Djabi, Y. Mely, and A.S. Klymchenko, Quantifying Release from Lipid Nanocarriers by Fluorescence Correlation Spectroscopy. ACS Omega, 2018. 3(10): p. 14333-14340.
124. Skilitsi, A.I., D. Agathangelou, I. Shulov, J. Conyard, S. Haacke, Y. Mely, A. Klymchenko and J. Leonard, Ultrafast photophysics of the environment-sensitive 4-methoxy-3-hydroxyflavone fluorescent dye. Physical Chemistry Chemical Physics, 2018. 20(11): p. 7885-7895.
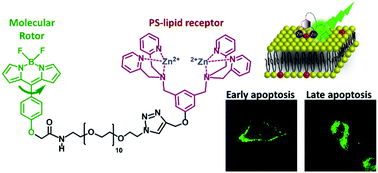
123. Pyrshev, K.A., A.S. Klymchenko, G. Csucs and A.P. Demchenko, Apoptosis and eryptosis: Striking differences on biomembrane level. Biochimica Et Biophysica Acta-Biomembranes, 2018. 1860(6): p. 1362-1371.
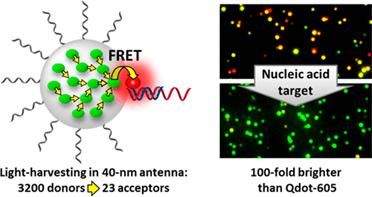
122. Melnychuk, N., and A.S. Klymchenko, DNA-Functionalized Dye-Loaded Polymeric Nanoparticles: Ultrabright FRET Platform for Amplified Detection of Nucleic Acids. Journal of the American Chemical Society, 2018. 140(34): p. 10856-10865.
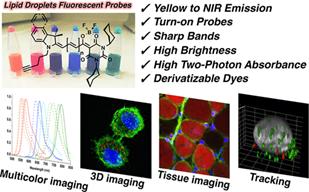
121. Fam, T.K., A.S. Klymchenko and M. Collot, Recent Advances in Fluorescent Probes for Lipid Droplets. Materials, 2018. 11(9): p. 1768.

120. Collot, M., T.K. Fam, P. Ashokkumar, O. Faklaris, T. Galli, L. Danglot and A.S. Klymchenko, Ultrabright and Fluorogenic Probes for Multicolor Imaging and Tracking of Lipid Droplets in Cells and Tissues. Journal of the American Chemical Society, 2018. 140(16): p. 5401-5411.
119. Cayre, F., S. Mura, B. Andreiuk, D. Sobot, S. Gouazou, D. Desmaele, A.S. Klymchenko and P. Couvreur, In Vivo FRET Imaging to Predict the Risk Associated with Hepatic Accumulation of Squalene-Based Prodrug Nanoparticles. Advanced Healthcare Materials, 2018. 7(3).
2017
118. Reisch, A., K. Trofymchuk , A. Runser, G. Fleith, M. Rawiso and A. S. Klymchenko, Tailoring Fluorescence Brightness and Switching of Nanoparticles through Dye Organization in the Polymer Matrix ACS Appl Mater Interfaces, 2017. 9(49): p. 43030–43042.
117. Trofymchuk, K., A. Reisch, P. Didier, F. Fras, P. Gilliot, Y. Mely and A.S. Klymchenko, Giant light-harvesting nanoantenna for single-molecule detection in ambient light. Nature Photonics, 2017. 11: p. 657-663.
116. Sobot, D., S. Mura, S.O. Yesylevskyy, L. Dalbin, F. Cayre, G. Bort, J. Mougin, D. Desmaele, S. Lepetre-Mouelhi, G. Pieters, B. Andreiuk, A.S. Klymchenko, J.L. Paul, C. Ramseyer, and P. Couvreur, Conjugation of squalene to gemcitabine as unique approach exploiting endogenous lipoproteins for drug delivery. Nature Communications, 2017. 8.
115. Snipstad, S., S. Hak, H. Baghirov, E. Sulheim, Y. Morch, S. Lelu, E. von Haartman, M. Back, K.P.R. Nilsson, A.S. Klymchenko, C.D. Davies, and A.K.O. Aslund, Labeling nanoparticles: Dye leakage and altered cellular uptake. Cytometry Part A, 2017. 91(8): p. 760-766.
114. Sherin, P.S., I. Lopez-Duarte, M.R. Dent, M. Kubankova, A. Vysniauskas, J.A. Bull, E.S. Reshetnikova, A.S. Klymchenko, Y.P. Tsentalovich and M.K. Kuimova, Visualising the membrane viscosity of porcine eye lens cells using molecular rotors. Chemical Science, 2017. 8(5): p. 3523-3528.
113. Sezgin, E., F. Schneider, V. Zilles, I. Urbancic, E. Garcia, D. Waithe, A.S. Klymchenko and C. Eggeling, Polarity-Sensitive Probes for Superresolution Stimulated Emission Depletion Microscopy. Biophysical Journal, 2017. 113(6): p. 1321-1330.
112. Roger, E., J.C. Gimel, C. Bensley, A.S. Klymchenko and J.P. Benoit, Lipid nanocapsules maintain full integrity after crossing a human intestinal epithelium model. Journal of Controlled Release, 2017. 253: p. 11-18.
111. Pyrshev, K.A., S.O. Yesylevskyy, Y. Mely, A.P. Demchenko and A.S. Klymchenko, Caspase-3 activation decreases lipid order in the outer plasma membrane leaflet during apoptosis: A fluorescent probe study. Biochimica Et Biophysica Acta-Biomembranes, 2017. 1859(10): p. 2123-2132.
110. Pivovarenko, V.G., O. Bugera, N. Humbert, A.S. Klymchenko and Y. Mely, A Toolbox of Chromones and Quinolones for Measuring a Wide Range of ATP Concentrations. Chemistry-a European Journal, 2017. 23(49): p. 11927-11934.
109. Kong, S.H., N. Haouchine, R. Soares, A. Klymchenko, B. Andreiuk, B. Marques, G. Shabat, T. Piechaud, M. Diana, S. Cotin, and J. Marescaux, Robust augmented reality registration method for localization of solid organs' tumors using CT-derived virtual biomechanical model and fluorescent fiducials. Surgical Endoscopy and Other Interventional Techniques, 2017. 31(7): p. 2863-2871.
108. Klymchenko, A.S., Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Accounts of Chemical Research, 2017. 50(2): p. 366-375.
107. Dukhno, O., F. Przybilla, M. Collot, A. Klymchenko, V. Pivovarenko, M. Buchner, V. Muhr, T. Hirsch and Y. Mely, Quantitative assessment of energy transfer in upconverting nanoparticles grafted with organic dyes. Nanoscale, 2017. 9(33): p. 11994-12004.
106. Ding, S., N. Anton, S. Akram, M. Er-Rafik, H. Anton, A. Klymchenko, W. Yu, T.F. Vandamme and C.A. Serra, A new method for the formulation of double nanoemulsions. Soft Matter, 2017. 13(8): p. 1660-1669.
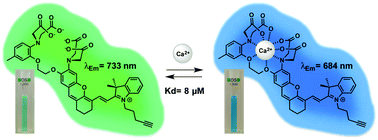
105. Collot, M., F. Ponsot and A.S. Klymchenko, Ca-NIR: a ratiometric near-infrared calcium probe based on a dihydroxanthene-hemicyanine fluorophore. Chemical Communications, 2017. 53(45): p. 6117-6120.
104. Bouchaala, R., N. Anton, H. Anton, T. Vandamme, J. Vermot, D. Smail, Y. Mely and A.S. Klymchenko, Light-triggered release from dye-loaded fluorescent lipid nanocarriers in vitro and in vivo. Colloids and Surfaces B-Biointerfaces, 2017. 156: p. 414-421.

103. Attia, M.F., S.M. Dieng, M. Collot, A.S. Klymchenko, C. Bouillot, C.A. Serra, M. Schmutz, M. Er-Rafik, T.F. Vandamme and N. Anton, Functionalizing Nanoemulsions with Carboxylates: Impact on the Biodistribution and Pharmacokinetics in Mice. Macromolecular Bioscience, 2017. 17(7).
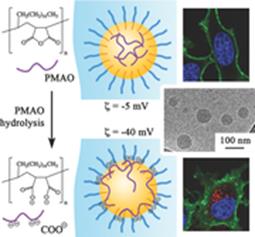
102. Andreiuk, B., A. Reisch, M. Lindecker, G. Follain, N. Peyrieras, J.G. Goetz and A.S. Klymchenko, Fluorescent Polymer Nanoparticles for Cell Barcoding In Vitro and In Vivo. Small, 2017. 13(38).
2016
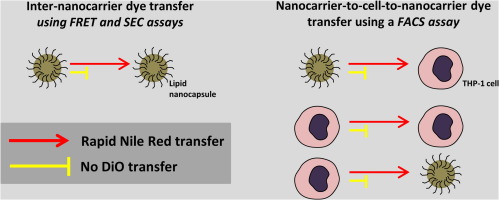
101. Simonsson, C., G. Bastiat, M. Pitorre, A.S. Klymchenko, J. Bejaud, Y. Mely and J.P. Benoit, Inter-nanocarrier and nanocarrier-to-cell transfer assays demonstrate the risk of an immediate unloading of dye from labeled lipid nanocapsules. European Journal of Pharmaceutics and Biopharmaceutics, 2016. 98: p. 47-56.
100. Shulov, I., R.V. Rodik, Y. Arntz, A. Reisch, V.I. Kalchenko and A.S. Klymchenko, Protein-Sized Bright Fluorogenic Nanoparticles Based on Cross-Linked Calixarene Micelles with Cyanine Corona. Angewandte Chemie-International Edition, 2016. 55(51): p. 15884-15888.
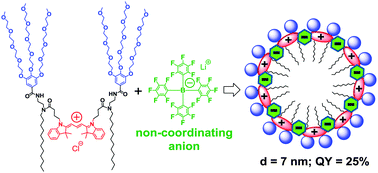
99. Shulov, I., Y. Arntz, Y. Mely, V.G. Pivovarenko and A.S. Klymchenko, Non-coordinating anions assemble cyanine amphiphiles into ultra-small fluorescent nanoparticles. Chemical Communications, 2016. 52(51): p. 7962-7965.
98. Reisch, A. and A.S. Klymchenko, Fluorescent Polymer Nanoparticles Based on Dyes: Seeking Brighter Tools for Bioimaging. Small, 2016. 12(15): p. 1968-1992.
97. Petrizza, L., M. Collot, L. Richert, Y. Mely, L. Prodi and A.S. Klymchenko, Dye-doped silica nanoparticle probes for fluorescence lifetime imaging of reductive environments in living cells. Rsc Advances, 2016. 6(106): p. 104164-104172.
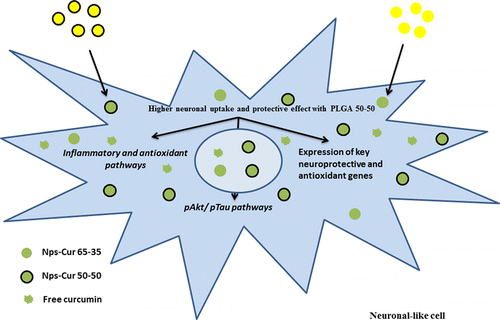
96. Paka, G.D., S. Doggui, A. Zaghmi, R. Safar, L. Dao, A. Reisch, A. Klymchenko, V.G. Roullin, O. Joubert and C. Ramassamy, Neuronal Uptake and Neuroprotective Properties of Curcumin-Loaded Nanoparticles on SK-N-SH Cell Line: Role of Poly(lactide-co-glycolide) Polymeric Matrix Composition. Molecular Pharmaceutics, 2016. 13(2): p. 391-403.
95. Niko, Y., P. Didier, Y. Mely, G. Konishi and A.S. Klymchenko, Bright and photostable push-pull pyrene dye visualizes lipid order variation between plasma and intracellular membranes. Scientific Reports, 2016. 6.
94. Karpenko, I.A., Y. Niko, V.P. Yakubovskyi, A.O. Gerasov, D. Bonnet, Y.P. Kovtun and A.S. Klymchenko, Push-pull dioxaborine as fluorescent molecular rotor: far-red fluorogenic probe for ligand-receptor interactions. Journal of Materials Chemistry C, 2016. 4(14): p. 3002-3009.
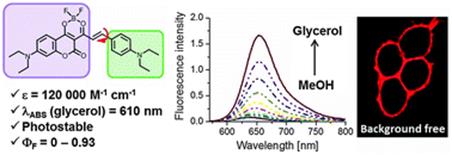
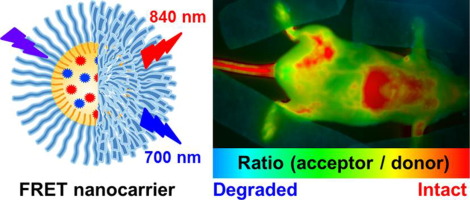
93. Bouchaala, R., L. Mercier, B. Andreiuk, Y. Mely, T. Vandamme, N. Anton, J.G. Goetz and A.S. Klymchenko, Integrity of lipid nanocarriers in bloodstream and tumor quantified by near-infrared ratiometric FRET imaging in living mice. Journal of Controlled Release, 2016. 236: p. 57-67.
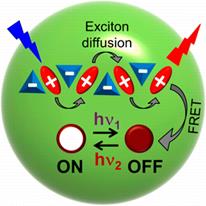
92. Trofymchuk, K., L. Prodi, A. Reisch, Y. Mely, K. Altenhoner, J. Mattay and A.S. Klymchenko, Exploiting Fast Exciton Diffusion in Dye-Doped Polymer Nanoparticles to Engineer Efficient Photoswitching. Journal of Physical Chemistry Letters, 2015. 6(12): p. 2259-2264.
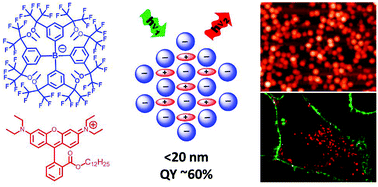
91. Shulov, I., S. Oncul, A. Reisch, Y. Arntz, M. Collot, Y. Mely and A.S. Klymchenko, Fluorinated counterion-enhanced emission of rhodamine aggregates: ultrabright nanoparticles for bioimaging and light-harvesting. Nanoscale, 2015. 7(43): p. 18198-18210.
90. Sholokh, M., O.M. Zamotaiev, R. Das, V.Y. Postupalenko, L. Richert, D. Dujardin, O.A. Zaporozhets, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Fluorescent Amino Acid Undergoing Excited State Intramolecular Proton Transfer for Site-Specific Probing and Imaging of Peptide Interactions. Journal of Physical Chemistry B, 2015. 119(6): p. 2585-2595.
2015
89. Rodik, R.V., A.S. Anthony, V.I. Kalchenko, Y. Mely and A.S. Klymchenko, Cationic amphiphilic calixarenes to compact DNA into small nanoparticles for gene delivery. New Journal of Chemistry, 2015. 39(3): p. 1654-1664.
88. Reisch, A., A. Runser, Y. Arntz, Y. Mely and A.S. Klymchenko, Charge-Controlled Nanoprecipitation as a Modular Approach to Ultrasmall Polymer Nanocarriers: Making Bright and Stable Nanoparticles. Acs Nano, 2015. 9(5): p. 5104-5116.
87. Neuberg, P., A. Perino, E. Morin-Picardat, N. Anton, Z. Darwich, D. Weltin, Y. Mely, A.S. Klymchenko, J.S. Remy and A. Wagner, Photopolymerized micelles of diacetylene amphiphile: physical characterization and cell delivery properties. Chemical Communications, 2015. 51(58): p. 11595-11598.
86. Kreder, R., K.A. Pyrshev, Z. Darwich, O.A. Kucherak, Y. Mely and A.S. Klymchenko, Solvatochromic Nile Red Probes with FRET Quencher Reveal Lipid Order Heterogeneity in Living and Apoptotic Cells. Acs Chemical Biology, 2015. 10(6): p. 1435-1442.
85. Kreder, R., S. Oncul, O.A. Kucherak, K.A. Pyrshev, E. Real, Y. Mely and A.S. Klymchenko, Blue fluorogenic probes for cell plasma membranes fill the gap in multicolour imaging. Rsc Advances, 2015. 5(29): p. 22899-22905.
84. Kilin, V., O. Glushonkov, L. Herdly, A. Klymchenko, L. Richert and Y. Mely, Fluorescence Lifetime Imaging of Membrane Lipid Order with a Ratiometric Fluorescent Probe. Biophysical Journal, 2015. 108(10): p. 2521-2531.
83. Khan, I.U., C.A. Serra, N. Anton, M. Er-Rafik, C. Blanck, M. Schmutz, I. Kraus, N. Messaddeq, C. Sutter, H. Anton, A.S. Klymchenko, and T.F. Vandamme, Microfluidic conceived Trojan microcarriers for oral delivery of nanoparticles. International Journal of Pharmaceutics, 2015. 493(1-2): p. 7-15.
82. Karpenko, I.A., A.S. Klymchenko, S. Gioria, R. Kreder, I. Shulov, P. Villa, Y. Mely, M. Hibert and D. Bonnet, Squaraine as a bright, stable and environment-sensitive far-red label for receptor-specific cellular imaging. Chemical Communications, 2015. 51(14): p. 2960-2963.
81. Karpenko, I.A., M. Collot, L. Richert, C. Valencia, P. Villa, Y. Mely, M. Hibert, D. Bonnet and A.S. Klymchenko, Fluorogenic Squaraine Dimers with Polarity-Sensitive Folding As Bright Far-Red Probes for Background-Free Bioimaging. Journal of the American Chemical Society, 2015. 137(1): p. 405-412.
80. Collot, M., R. Kreder, A.L. Tatarets, L.D. Patsenker, Y. Mely and A.S. Klymchenko, Bright fluorogenic squaraines with tuned cell entry for selective imaging of plasma membrane vs. endoplasmic reticulum. Chemical Communications, 2015. 51(96): p. 17136-17139.
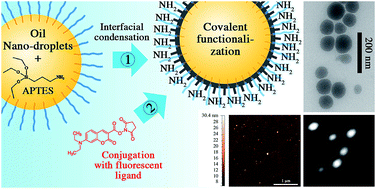
79. Attia, M.F., N. Anton, R. Bouchaala, P. Didier, Y. Arntz, N. Messaddeq, A.S. Klymchenko, Y. Mely and T.F. Vandamme, Functionalization of nano-emulsions with an amino-silica shell at the oil-water interface. Rsc Advances, 2015. 5(91): p. 74353-74361.
2014
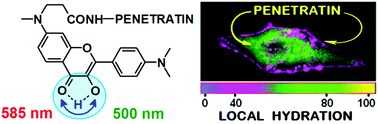
78. Zamotaiev, O.M., V.Y. Postupalenko, V.V. Shvadchak, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Monitoring penetratin interactions with lipid membranes and cell internalization using a new hydration-sensitive fluorescent probe. Organic & Biomolecular Chemistry, 2014. 12(36): p. 7036-7044.
77. van Hell, A.J., A. Klymchenko, D.M. Gueth, W.J. van Blitterswijk, G.A. Koning and M. Verheij, Membrane organization determines barrier properties of endothelial cells and short-chain sphingolipid-facilitated doxorubicin influx. Biochimica Et Biophysica Acta-Molecular and Cell Biology of Lipids, 2014. 1841(9): p. 1301-1307.
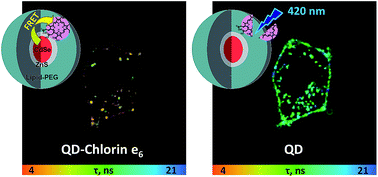
76. Valanciunaite, J., A.S. Klymchenko, A. Skripka, L. Richert, S. Steponkiene, G. Streckyte, Y. Mely and R. Rotomskis, A non-covalent complex of quantum dots and chlorin e(6): efficient energy transfer and remarkable stability in living cells revealed by FLIM. Rsc Advances, 2014. 4(94): p. 52270-52278.
75. Trofymchuk, K., A. Reisch, I. Shulov, Y. Mely and A.S. Klymchenko, Tuning the color and photostability of perylene diimides inside polymer nanoparticles: towards biodegradable substitutes of quantum dots. Nanoscale, 2014. 6(21): p. 12934-12942.
74. Saxena, R., S. Shrivastava, S. Haldar, A.S. Klymchenko and A. Chattopadhyay, Location, dynamics and solvent relaxation of a nile red-based phase-sensitive fluorescent membrane probe. Chemistry and Physics of Lipids, 2014. 183: p. 1-8.
73. Sasaki, S., Y. Niko, A.S. Klymchenko and G.I. Konishi, Design of donor-acceptor geometry for tuning excited-state polarization: fluorescence solvatochromism of push-pull biphenyls with various torsional restrictions on their aryl-aryl bonds. Tetrahedron, 2014. 70(41): p. 7551-7559.
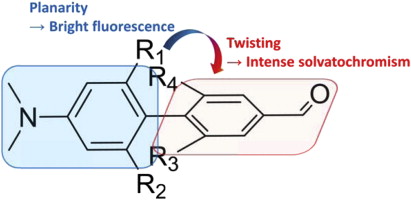
72. Rodik, R.V., A.S. Klymchenko, Y. Mely and V.I. Kalchenko, Calixarenes and related macrocycles as gene delivery vehicles. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 2014. 80(3-4): p. 189-200.
71. Reisch, A., P. Didier, L. Richert, S. Oncul, Y. Arntz, Y. Mely and A.S. Klymchenko, Collective fluorescence switching of counterion-assembled dyes in polymer nanoparticles. Nature Communications, 2014. 5.
70. Niko, Y., Y. Arntz, Y. Mely, G. Konishi and A.S. Klymchenko, Disassembly-Driven Fluorescence Turn-on of Polymerized Micelles by Reductive Stimuli in Living Cells. Chemistry-a European Journal, 2014. 20(50): p. 16473-16477.
69. Mely, Y., V.Y. Postupalenko, V.V. Shvadchak, M. Sholokh, A.S. Klymchenko, A.V. Strizhak, O.M. Zamotaiev and V.G. Pivovarenko, Development of ESIPT-based fluorescent L-amino acid analogs: Applications in monitoring protein/ligand interactions. Abstracts of Papers of the American Chemical Society, 2014. 248.
68. Mely, Y., V. Kilin, O. Glushonkov, L. Richert and A.S. Klymchenko, ESIPT-based fluorescent probe for imaging lipid domains in membranes. Abstracts of Papers of the American Chemical Society, 2014. 248.
67. Klymchenko, A.S. and R. Kreder, Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chemistry & Biology, 2014. 21(1): p. 97-113.
66. Kilin, V.N., H. Anton, N. Anton, E. Steed, J. Vermot, T.E. Vandamme, Y. Mely and A.S. Klymchenko, Counterion-enhanced cyanine dye loading into lipid nano-droplets for single-particle tracking in zebrafish. Biomaterials, 2014. 35(18): p. 4950-4957.
65. Karpenko, I.A., R. Kreder, C. Valencia, P. Villa, C. Mendre, B. Mouillac, Y. Mely, M. Hibert, D. Bonnet and A.S. Klymchenko, Red Fluorescent Turn-On Ligands for Imaging and Quantifying G Protein-Coupled Receptors in Living Cells. Chembiochem, 2014. 15(3): p. 359-363.
64. Jakhmola, A., N. Anton, H. Anton, N. Messaddeq, F. Hallouard, A. Klymchenko, Y. Mely and T.F. Vandamme, Poly-epsilon-caprolactone tungsten oxide nanoparticles as a contrast agent for X-ray computed tomography. Biomaterials, 2014. 35(9): p. 2981-2986.
63. Greiner, V.J., C. Manin, E. Larquet, N. Ikhelef, F. Greco, S. Naville, P.E. Milhiet, F. Ronzon, A. Klymchenko and Y. Mely, Characterization of the structural modifications accompanying the loss of HBsAg particle immunogenicity. Vaccine, 2014. 32(9): p. 1049-1054.
62. Dziuba, D., I.A. Karpenko, N.P.F. Barthes, B.Y. Michel, A.S. Klymchenko, R. Benhida, A.P. Demchenko, Y. Mely and A. Burger, Rational Design of a Solvatochromic Fluorescent Uracil Analogue with a Dual-Band Ratiometric Response Based on 3-Hydroxychromone. Chemistry-a European Journal, 2014. 20(7): p. 1998-2009.
61. Das, R., G. Duportail, A. Ghose, L. Richert, A. Klymchenko, S. Chakraborty, S. Yesylevskyy and Y. Mely, Tuning excited-state proton transfer dynamics of a 3-hydroxychromone dye in supramolecular complexes via host-guest steric compatibility. Physical Chemistry Chemical Physics, 2014. 16(2): p. 776-784.
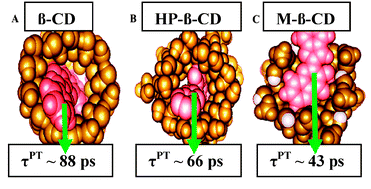
60. Darwich, Z., A.S. Klymchenko, D. Dujardin and Y. Mely, Imaging lipid order changes in endosome membranes of live cells by using a Nile Red-based membrane probe. Rsc Advances, 2014. 4(17): p. 8481-8488.
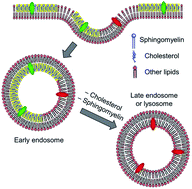
59. Boisrame-Helms, J., X. Delabranche, A. Klymchenko, J. Drai, E. Blond, F. Zobairi, Y. Mely, M. Hasselmann, F. Toti and F. Meziani, Lipid Emulsions Differentially Affect LPS-Induced Acute Monocytes Inflammation: In Vitro Effects on Membrane Remodeling and Cell Viability. Lipids, 2014. 49(11): p. 1091-1099.
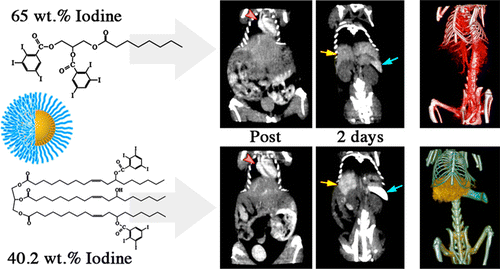
58. Attia, M.F., N. Anton, M. Chiper, R. Akasov, H. Anton, N. Messaddeq, S. Fournel, A.S. Klymchenko, Y. Mely and T.F. Vandamme, Biodistribution of X-Ray Iodinated Contrast Agent in Nano-Emulsions Is Controlled by the Chemical Nature of the Oily Core. Acs Nano, 2014. 8(10): p. 10537-10550.
2013
57. Ziomkiewicz, I., A. Loman, R. Klement, C. Fritsch, A.S. Klymchenko, G. Bunt, T.M. Jovin and D.J. Arndt-Jovin, Dynamic conformational transitions of the EGF receptor in living mammalian cells determined by FRET and fluorescence lifetime imaging microscopy. Cytometry Part A, 2013. 83(9): p. 794-805.
56. Prodanov, M.F., N.V. Pogorelova, A.P. Kryshtal, A.S. Klymchenko, Y. Mely, V.P. Semynozhenko, A.I. Krivoshey, Y.A. Reznikov, S.N. Yarmolenko, J.W. Goodby, and V.V. Vashchenko, Thermodynamically Stable Dispersions of Quantum Dots in a Nematic Liquid Crystal. Langmuir, 2013. 29(30): p. 9301-9309.
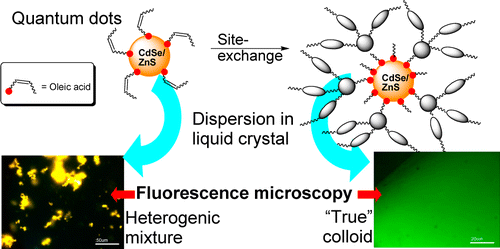
55. Postupalenko, V.Y., O.M. Zamotaiev, V.V. Shvadchak, A.V. Strizhak, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Dual-Fluorescence L-Amino Acid Reports Insertion and Orientation of Melittin Peptide in Cell Membranes. Bioconjugate Chemistry, 2013. 24(12): p. 1998-2007.
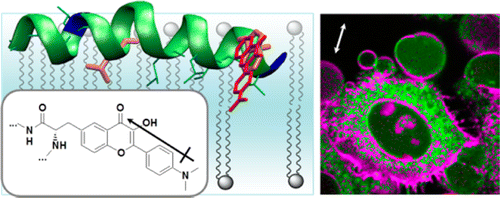
54. Klymchenko, A.S. and Y. Mely, Fluorescent Environment-Sensitive Dyes as Reporters of Biomolecular Interactions. Fluorescence-Based Biosensors: From Concepts to Applications, 2013. 113: p. 35-58.
53. Darwich, Z., O.A. Kucherak, R. Kreder, L. Richert, R. Vauchelles, Y. Mely and A.S. Klymchenko, Rational design of fluorescent membrane probes for apoptosis based on 3-hydroxyflavone. Methods and Applications in Fluorescence, 2013. 1(2).
2012
52. Strizhak, A.V., V.Y. Postupalenko, V.V. Shvadchak, N. Morellet, E. Guittet, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Two-Color Fluorescent L-Amino Acid Mimic of Tryptophan for Probing Peptide-Nucleic Acid Complexes. Bioconjugate Chemistry, 2012. 23(12): p. 2434-2443.
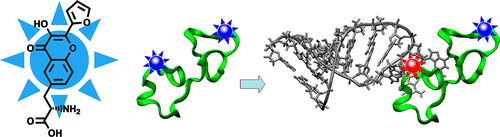
51. Pivovarenko, V.G., O.M. Zamotaiev, V.V. Shvadchak, V.Y. Postupalenko, A.S. Klymchenko and Y. Mely, Quantification of Local Hydration at the Surface of Biomolecules Using Dual-Fluorescence Labels. Journal of Physical Chemistry A, 2012. 116(12): p. 3103-3109.
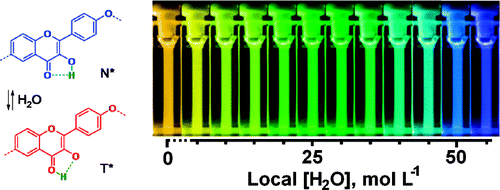
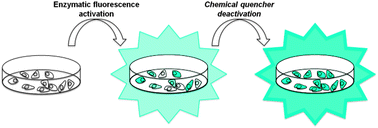
50. Leriche, G., G. Budin, Z. Darwich, D. Weltin, Y. Mely, A.S. Klymchenko and A. Wagner, A FRET-based probe with a chemically deactivatable quencher. Chemical Communications, 2012. 48(26): p. 3224-3226.
 <
<
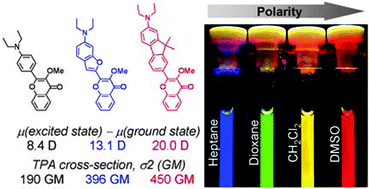
49. Kucherak, O.A., L. Richert, Y. Mely and A.S. Klymchenko, Dipolar 3-methoxychromones as bright and highly solvatochromic fluorescent dyes. Physical Chemistry Chemical Physics, 2012. 14(7): p. 2292-2300.
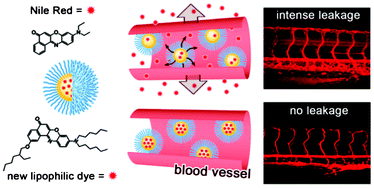
48. Klymchenko, A.S., E. Roger, N. Anton, H. Anton, I. Shulov, J. Vermot, Y. Mely and T.F. Vandamme, Highly lipophilic fluorescent dyes in nano-emulsions: towards bright non-leaking nano-droplets. Rsc Advances, 2012. 2(31): p. 11876-11886.
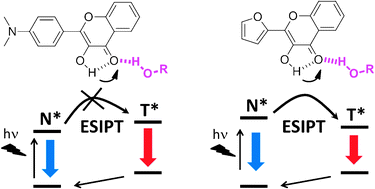
47. Klymchenko, A.S., Solvatochromic fluorescent dyes as universal tools for biological research. Actualite Chimique, 2012(359): p. 20-26.
46. Kenfack, C.A., A.S. Klymchenko, G. Duportail, A. Burger and Y. Mely, Ab initio study of the solvent H-bonding effect on ESIPT reaction and electronic transitions of 3-hydroxychromone derivatives. Physical Chemistry Chemical Physics, 2012. 14(25): p. 8910-8918.
45. Jain, N., V. Goldschmidt, S. Oncul, Y. Arntz, G. Duportail, Y. Mely and A.S. Klymchenko, Lactose-ornithine bolaamphiphiles for efficient gene delivery in vitro. International Journal of Pharmaceutics, 2012. 423(2): p. 392-400.
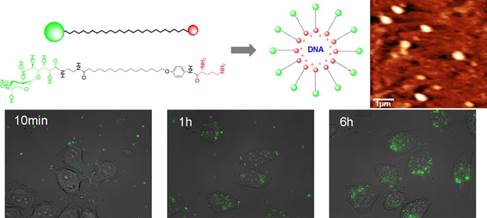
44. Greiner, V.J., F. Ronzon, E. Larquet, B. Desbat, C. Esteves, J. Bonvin, F. Greco, C. Manin, A.S. Klymchenko and Y. Mely, The structure of HBsAg particles is not modified upon their adsorption on aluminium hydroxide gel. Vaccine, 2012. 30(35): p. 5240-5245.
43. Dziuba, D., V.Y. Postupalenko, M. Spadafora, A.S. Klymchenko, V. Guerineau, Y. Mely, R. Benhida and A. Burger, A Universal Nucleoside with Strong Two-Band Switchable Fluorescence and Sensitivity to the Environment for Investigating DNA Interactions. Journal of the American Chemical Society, 2012. 134(24): p. 10209-10213.
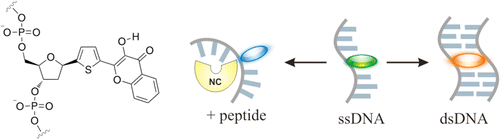
42. Das, R., G. Duportail, L. Richert, A. Klymchenko and Y. Mely, Sensing Micelle Hydration by Proton-Transfer Dynamics of a 3-Hydroxychromone Dye: Role of the Surfactant Headgroup and Chain Length. Langmuir, 2012. 28(18): p. 7147-7159.
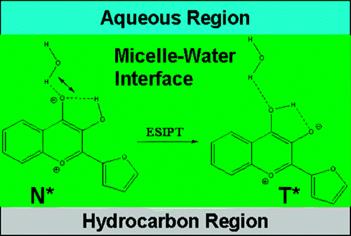
41. Darwich, Z., A.S. Klymchenko, O.A. Kucherak, L. Richert and Y. Mely, Detection of apoptosis through the lipid order of the outer plasma membrane leaflet. Biochimica Et Biophysica Acta-Biomembranes, 2012. 1818(12): p. 3048-3054.
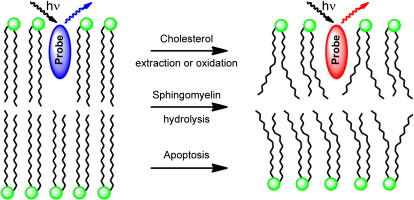
40. Chiantia, S., A.S. Klymchenko and E. London, A novel leaflet-selective fluorescence labeling technique reveals differences between inner and outer leaflets at high bilayer curvature. Biochimica Et Biophysica Acta-Biomembranes, 2012. 1818(5): p. 1284-1290.
2011
39. Zamotaiev, O.M., V.Y. Postupalenko, V.V. Shvadchak, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Improved Hydration-Sensitive Dual-Fluorescence Labels For Monitoring Peptide-Nucleic Acid Interactions. Bioconjugate Chemistry, 2011. 22(1): p. 101-107.
38. Rodik, R.V., A.S. Klymchenko, N. Jain, S.I. Miroshnichenko, L. Richert, V.I. Kalchenko and Y. Mely, Virus-Sized DNA Nanoparticles for Gene Delivery Based on Micelles of Cationic Calixarenes. Chemistry-a European Journal, 2011. 17(20): p. 5526-5538.
37. Postupalenko, V.Y., V.V. Shvadchak, G. Duportail, V.G. Pivovarenko, A.S. Klymchenko and Y. Mely, Monitoring membrane binding and insertion of peptides by two-color fluorescent label. Biochimica Et Biophysica Acta-Biomembranes, 2011. 1808(1): p. 424-432.
36. Perino, A., A. Klymchenko, A. Morere, E. Contal, A. Rameau, J.M. Guenet, Y. Mely and A. Wagner, Structure and Behavior of Polydiacetylene-Based Micelles. Macromolecular Chemistry and Physics, 2011. 212(2): p. 111-117.
35. Contal, E., A.S. Klymchenko, Y. Mely, S. Meunier and A. Wagner, Core functionalization of polydiacetylene micelles by a "click" reaction. Soft Matter, 2011. 7(5): p. 1648-1650.
34. Chiantia, S., P. Schwille, A.S. Klymchenko and E. London, Asymmetric GUVs Prepared by M beta CD-Mediated Lipid Exchange: An FCS Study. Biophysical Journal, 2011. 100(1): p. Lo1-Lo3
2010
33. van Hell, A.J., A. Klymchenko, P.P. Burgers, E.E. Moret, W. Jiskoot, W.E. Hennink, D.J.A. Crommelin and E. Mastrobattista, Conformation and Intermolecular Interactions of SA2 Peptides Self-Assembled into Vesicles. Journal of Physical Chemistry B, 2010. 114(34): p. 11046-11052.
32. Szczupak, B., A.G. Ryder, D.M. Togashi, A.S. Klymchenko, Y.A. Rochev, A. Gorelov and T.J. Glynn, Polarity Assessment of Thermoresponsive Poly(NIPAM-co-NtBA) Copolymer Films Using Fluorescence Methods. Journal of Fluorescence, 2010. 20(3): p. 719-731.
31. Roche, Y., A.S. Klymchenko, P. Gerbeau-Pissot, P. Gervais, Y. Mely, F. Simon-Plas and J.M. Perrier-Cornet, Behavior of plant plasma membranes under hydrostatic pressure as monitored by fluorescent environment-sensitive probes. Biochimica Et Biophysica Acta-Biomembranes, 2010. 1798(8): p. 1601-1607.
30. Oncul, S., A.S. Klymchenko, O.A. Kucherak, A.P. Demchenko, S. Martin, M. Dontenwill, Y. Arntz, P. Didier, G. Duportail and Y. Mely, Liquid ordered phase in cell membranes evidenced by a hydration-sensitive probe: Effects of cholesterol depletion and apoptosis. Biochimica Et Biophysica Acta-Biomembranes, 2010. 1798(7): p. 1436-1443.
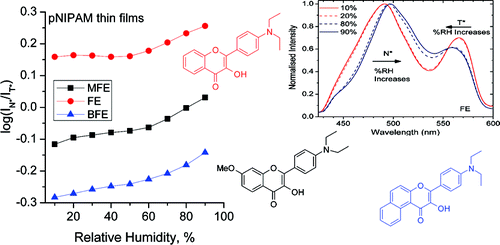
29. Morris, C., B. Szczupak, A.S. Klymchenko and A.G. Ryder, Study of Water Adsorption in Poly(N-isopropylacrylamide) Thin Films Using Fluorescence Emission of 3-Hydroxyflavone Probes. Macromolecules, 2010. 43(22): p. 9488-9494.
28. Kucherak, O.A., S. Oncul, Z. Darwich, D.A. Yushchenko, Y. Arntz, P. Didier, Y. Mely and A.S. Klymchenko, Switchable Nile Red-Based Probe for Cholesterol and Lipid Order at the Outer Leaflet of Biomembranes. Journal of the American Chemical Society, 2010. 132(13): p. 4907-4916.
27. Kucherak, O.A., P. Didier, Y. Mely and A.S. Klymchenko, Fluorene Analogues of Prodan with Superior Fluorescence Brightness and Solvatochromism. Journal of Physical Chemistry Letters, 2010. 1(3): p. 616-620.
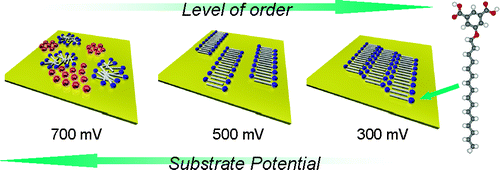
26. Klymchenko, A.S., S. Furukawa, T. Balandina, K. Mullen, M. Van der Auweraer and S. De Feyter, 2D analogues of the inverted hexagonal phase self-assembled from 4,6-dialkoxylated isophthalic acids at solid-liquid interfaces. Nanoscale, 2010. 2(9): p. 1773-1780.
25. Jain, N., Y. Arntz, V. Goldschmidt, G. Duportail, Y. Mely and A.S. Klymchenko, New Unsymmetrical Bolaamphiphiles: Synthesis, Assembly with DNA, and Application for Gene Delivery. Bioconjugate Chemistry, 2010. 21(11): p. 2110-2118.
24. Greiner, V.J., C. Egele, S. Oncul, F. Ronzon, C. Manin, A. Klymchenko and Y. Mely, Characterization of the lipid and protein organization in HBsAg viral particles by steady-state and time-resolved fluorescence spectroscopy. Biochimie, 2010. 92(8): p. 994-1002.
23. Choulier, L., V.V. Shvadchak, A. Naidoo, A.S. Klymchenko, Y. Mely and D. Altschuh, A peptide-based fluorescent ratiometric sensor for quantitative detection of proteins. Analytical Biochemistry, 2010. 401(2): p. 188-195.
2009
22. Spadafora, M., V.Y. Postupalenko, V.V. Shvadchak, A.S. Klymchenko, Y. Mely, A. Burger and R. Benhida, Efficient Synthesis of Ratiometric Fluorescent Nucleosides Featuring 3-Hydroxychromone Nucleobases. Tetrahedron, 2009. 65(37): p. 7809-7816.
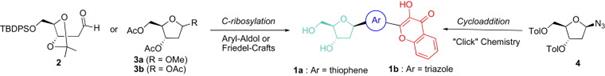
21. Shvadchak, V.V., A.S. Klymchenko, H. de Rocquigny and Y. Mely, Sensing peptide-oligonucleotide interactions by a two-color fluorescence label: application to the HIV-1 nucleocapsid protein. Nucleic Acids Research, 2009. 37(3).
20. Klymchenko, A.S., S. Oncul, P. Didier, E. Schaub, L. Bagatolli, G. Duportail and Y. Mely, Visualization of lipid domains in giant unilamellar vesicles using an environment-sensitive membrane probe based on 3-hydroxyflavone. Biochimica Et Biophysica Acta-Biomembranes, 2009. 1788(2): p. 495-499.
19. Demchenko, A.P., Y. Mely, G. Duportail and A.S. Klymchenko, Monitoring Biophysical Properties of Lipid Membranes by Environment-Sensitive Fluorescent Probes. Biophysical Journal, 2009. 96(9): p. 3461-3470.
18. Das, R., A.S. Klymchenko, G. Duportail and Y. Mely, Unusually slow proton transfer dynamics of a 3-hydroxychromone dye in protic solvents. Photochemical & Photobiological Sciences, 2009. 8(11): p. 1583-1589.
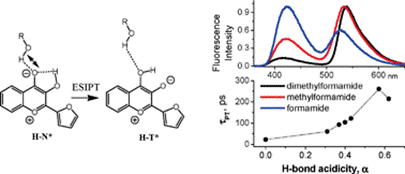
17. Boudier, C., A.S. Klymchenko, Y. Mely and A. Follenius-Wund, Local environment perturbations in alpha(1)-antitrypsin monitored by a ratiometric fluorescent label. Photochemical & Photobiological Sciences, 2009. 8(6): p. 814-821.
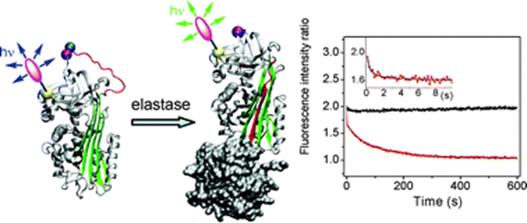
16. Bilokin, M.D., V.V. Shvadchak, D.A. Yushchenko, A.S. Klymchenko, G. Duportail, Y. Mely and V.G. Pivovarenko, 3-Hydroxybenzo[g]quinolones: dyes with red-shifted absorption and highly resolved dual emission. Tetrahedron Letters, 2009. 50(33): p. 4714-4719.
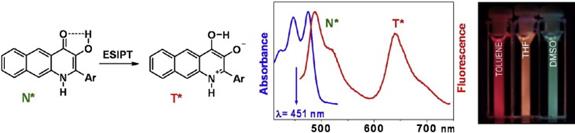
2008
15. Wang, G.J., M. Abramov, A. Van Aerschot, J. Rozenski, S.B. Lei, A. Klymchenko, M. Van der Auweraer, S. De Feyter and P. Herdewijn, Dendritic nucleotides: Interaction with an aliphatic acid monolayer. Chemistry & Biodiversity, 2008. 5(9): p. 1675-1682.
14. M'Baye, G., Y. Mely, G. Duportail and A.S. Klymchenko, Liquid ordered and gel phases of lipid bilayers: Fluorescent probes reveal close fluidity but different hydration. Biophysical Journal, 2008. 95(3): p. 1217-1225.
13. Klymchenko, A.S., V.V. Shvadchak, D.A. Yushchenko, N. Jain and Y. Mely, Excited-state intramolecular proton transfer distinguishes microenvironments in single- and double-stranded DNA. Journal of Physical Chemistry B, 2008. 112(38): p. 12050-12055.
12. Klymchenko, A.S., S. Furukawa, M. Van der Auweraer, K. Mullen and S. De Feyter, Directing the assembly of charged organic molecules by a hydrophilic-hydrophobic nanostructured monolayer at electrified interfaces. Nano Letters, 2008. 8(4): p. 1163-1168.
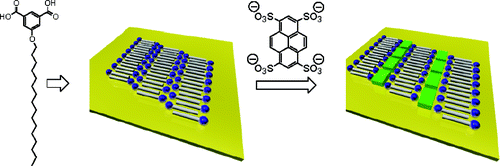
11. Klymchenko, A.S. and A.R. Demchenko, Multiparametric Probing of Microenvironment with Solvatochromic Fluorescent Dyes. Fluorescence Spectroscopy, 2008. 450: p. 37-58.
10. Enander, K., L. Choulier, A.L. Olsson, D.A. Yushchenko, D. Kanmert, A.S. Klymchenko, A.P. Demchenko, Y. Mely and D. Altschuh, A peptide-based, ratiometric biosensor construct for direct fluorescence detection of a protein analyte. Bioconjugate Chemistry, 2008. 19(9): p. 1864-1870.
9. Das, R., A.S. Klymchenko, G. Duportail and Y. Mely, Excited state proton transfer and solvent relaxation of a 3-hydroxyflavone probe in lipid bilayers. Journal of Physical Chemistry B, 2008. 112(38): p. 11929-11935.
2007
8. Yushchenko, D.A., V.V. Shvadchak, A.S. Klymchenko, G. Duportail, V.G. Pivovarenko and Y. Mely, Steric control of the excited-state intramolecular proton transfer in 3-hydroxyquinolones: Steady-state and time-resolved fluorescence study. Journal of Physical Chemistry A, 2007. 111(37): p. 8986-8992.
7. Yushchenko, D.A., V.V. Shvadchak, A.S. Klymchenko, G. Duportail, V.G. Pivovarenko and Y. Mely, Modulation of excited-state intramolecular proton transfer by viscosity in protic media. Journal of Physical Chemistry A, 2007. 111(42): p. 10435-10438.
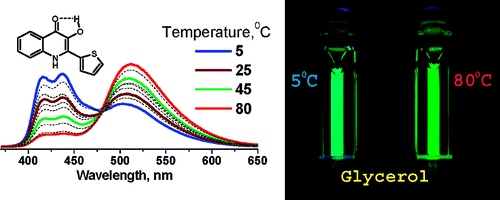
6. Shynkar, V.V., A.S. Klymchenko, C. Kunzelmann, G. Duportail, C.D. Muller, A.P. Demchenko, J.M. Freyssinet and Y. Mely, Fluorescent biomembrane probe for ratiometric detection of apoptosis. Journal of the American Chemical Society, 2007. 129(7): p. 2187-2193.
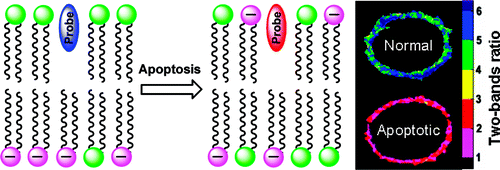
5. Ozturk, T., A.S. Klymchenko, A. Capan, S. Oncul, S. Cikrikci, S. Taskiran, B. Tasan, F.B. Kaynak, S. Ozbey and A.P. Demchenko, New 3-hydroxyflavone derivatives for probing hydrophobic sites in microheterogeneous systems. Tetrahedron, 2007. 63(41): p. 10290-10299.
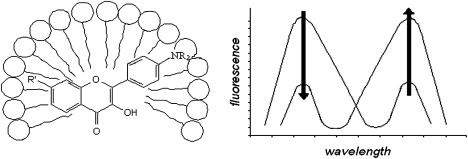
4. M'Baye, G., A.S. Klymchenko, D.A. Yushchenko, V.V. Shvadchak, T. Ozturk, Y. Mely and G. Duportail, Fluorescent dyes undergoing intramolecular proton transfer with improved sensitivity to surface charge in lipid bilayers. Photochemical & Photobiological Sciences, 2007. 6(1): p. 71-76.
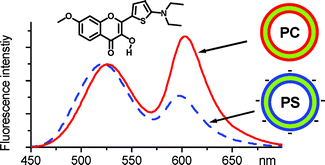
3. Klymchenko, A.S., D.A. Yushchenko and Y. Mely, Tuning excited state intramolecular proton transfer in 3-hydroxyflavone derivative by reaction of its isothiocyanate group with an amine. Journal of Photochemistry and Photobiology a-Chemistry, 2007. 192(2-3): p. 93-97.
2. Klymchenko, A.S., C. Kenfack, G. Duportail and Y. Mely, Effects of polar protic solvents on dual emissions of 3-hydroxychromones. Journal of Chemical Sciences, 2007. 119(2): p. 83-89.
1. Klymchenko, A.S., S. Furukawa, K. Mullen, M. Van der Auweraer and S. De Feyter, Supramolecular hydrophobic-hydrophilic nanopatterns at electrified interfaces. Nano Letters, 2007. 7(3): p. 791-795.