Partenaires


Rechercher
 |
|||||
|
Fluorescent molecular probes Solvatochromic and fluorogenic dyes Fluorescent environment-sensitive probes are specially designed dyes that change their fluorescence intensity (fluorogenic) or color (e.g. solvatochromic) in response to change in their microenvironment polarity, viscosity and molecular order.1 The studies of the last decade, including those of our group, have shown that these molecules become universal tools in fluorescence sensing and imaging. In fact, any biomolecular interaction or change in biomolecular organization results in modification of the local microenvironment, which can be directly monitored by these types of probes. References
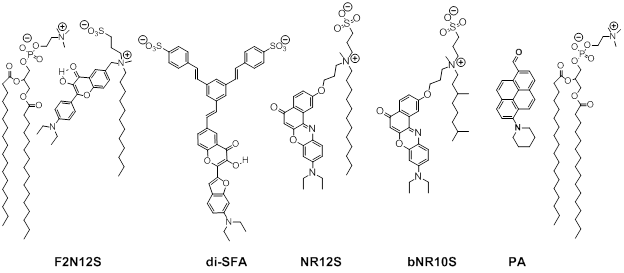
Fig. 2. Examples of environment-sensitive membrane probes developed in our group. Probe PA was developed together with Yosuke Niko (Japan). We develop new molecular probes sensitive to different properties of lipid membranes. The selectivity of the probes to a particular membrane property can be achieved by proper selection of the fluorophore and its appropriate localization in the lipid membrane 3. Representative examples are probes of hydration and surface charge, F2N12S,1 transmembrane potential, di-SFA 2 and lipid order: F2N12S 1, NR12S 3, bNR10S 4and PA 5.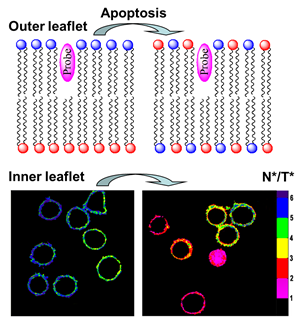 Fig. 3. Response of a polarity-sensitive membrane probe F2N12S to apoptotic changes in cell plasma membranes. Changes in the ratio of two emission bands of F2N12S on apoptosis in CEM cells imaged by confocal microscopy. Data adapted from 1. Normal cell membranes exhibit strong transbilayer asymmetry, while in apoptotic cells, it is lost. In our earlier work in 2007, within a group of Yves Mely (UMR7213, Strasbourg), we developed F2N12S, which incorporates selectively into the outer leaflet of the cell plasma membrane, can detect this loss of asymmetry by changing its N*/T* intensity ratio.1 Being ratiometric, the response of the new probe can be easily quantified by microscopy techniques, a feature that can be hardly achieved with the commonly used fluorescently labeled annexin V. Later on, we showed that NR12S can also be applied for apoptosis detection.6Probes for lipid order and microdomains 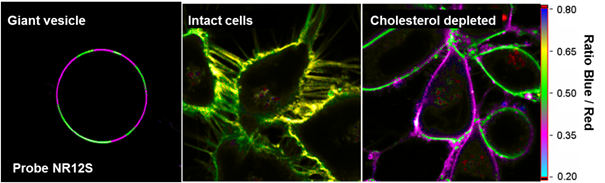 Fig. 4. Ratiometric imaging of giant vesicles composed of Lo and Ld domains as visualized by NR12S (left). Intact cells and cells after cholesterol extraction with MbCD showing different color in the ratiometric images (middle and right). Two-photon excitation at 830 nm was used. Sizes of the images were 50´50 mm. Data from Ref.3 Membrane domains or “rafts” are believed to be involved in various cell functions. In model membranes, these domains, defined as liquid-ordered (Lo) phase enriched in cholesterol (Chol) and sphingomyelin (SM), can be observed as “rafts” floating in the liquid disordered (Ld) phase, enriched in unsaturated phospholipids (DOPC). According to our earlier work in 2010, probe NR12S due to its high sensitivity to the membrane hydration allows visualization of lipid domains in model membranes and monitoring lipid order in cellular membranes using ratiometric imaging (Fig. 4).3 Later on, we developed a bulky version of NR12S, so-called bNR10S, and BHQ-2 based quencher, which showed specific partitioning to Ld phase. These probes enabled direct demonstration of phase heterogeneity in plasma membrane of living cells.4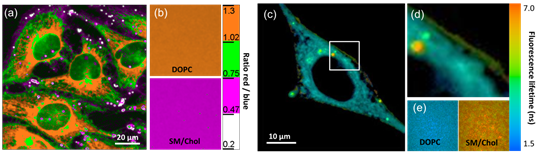 Fig. 5. Ratiometric (a-b) and FLIM (c-e) imaging of lipid order in living HeLa cells using solvatochromic probe PA. The pseudo-colors represent the ratio of the long- to short-wavelength emission channels (550-700 nm to 470-550 nm). (b) Calibration of the ratio using suspension of lipid vesicles representing Ld (DOPC) and Lo (SM/Cho, Lo phase) phases. (c) FLIM image of HeLa cells and (d) zoom on the region of interest. (e) Calibration images of suspensions of vesicles. Adapted from 5. In more recent studies, together with Yosuke Niko, JSPS post-doctoral fellow in our group, we showed that push-pull pyrene (PA, Fig. 2), similarly to Laurdan, changes the emission maximum as a function of lipid order, but outperforms it by spectroscopic properties.5 In addition to red-shifted absorption compatible with 405-nm diode laser, PA shows higher brightness and much higher photostability than Laurdan. Moreover, PA is compatible with two-photon excitation at wavelengths >800 nm, which was successfully used for ratiometric imaging of coexisting Lo and Ld phases. PA efficiently stains the plasma membrane and the intracellular membranes at >20-fold lower concentrations, as compared to Laurdan. Finally, ratiometric imaging and FLIM using PA revealed variation of lipid order within different cellular compartments: plasma membranes are close to Lo phase, while intracellular membranes are much less ordered, reflecting lower cholesterol content (Fig. 5).5 PA probe constitutes thus a new powerful tool for biomembrane research. Our ongoing work on development of membrane probes revealed the other important aspect – the need in the bright probes that can specifically stain the cell plasma membranes in a desired color. In this case, one needs to use fluorophores with high brightness and photostability, exhibiting narrow absorption and emission bands in the regions complementary to commonly used fluorescent proteins. Here, we considered two blue and far red spectral regions. Multi-color imaging needs these probes, because of their complementary with green and red fluorescent proteins. Using 3-methoxychromone dyes, we designed two blue membrane probes F2N12SM and FC12SM (Fig. 6) exhibiting >100-fold fluorescence turn-on (fluorogenic response) on membrane binding, large Stokes shift (70–90 nm) as well as high brightness and photostability.7 These unique properties enabled cellular imaging at low probe concentrations (20–50 nM) with minimal background from cell auto-fluorescence and from free probe. RGB multicolour imaging was successfully realized using these probes in combination with common green and red markers (Fig. 6). These new blue probes may fill the gap in multi-color microscopy.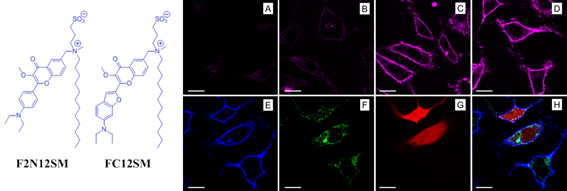 Fig. 6. (Left) Chemical structures of the new membrane probes, F2N12SM and FC12SM. (Right) Fluorescence confocal images of HeLa cells without probes (A) and stained with F2N12S (B), F2N12SM (C) or FC12SM (D) at 50 nM concentrations. (E-H) Multicolour confocal imaging using F2N12SM (blue, E), LysoTracker® Green DND-26 (green, F) and mCherry (red, G) and merge of the three images (H). Scale bar is 20 µm. Adapted from.4 To obtain bright far-red membrane probes we focused on squaraines, dyes exhibiting exceptional extinction coefficient (~300,000 M-1 cm-1) as well as high quantum yield and photostability. We designed three amphiphilic squaraine probes containing hydrocarbon chains and zwitterionic polar head groups.8 We found that SQ8S quickly crossed the plasma membrane to efficiently stain the endoplasmic reticulum membranes (Fig. 4). SQ12S distributeed in both plasma and ER membranes (Fig. 4). Finally, dSQ12S, due to its two anchors, stained specifically plasma membranes without significant internalization (Fig. 4). Remarkably, we were able to obtain high quality confocal images even at 1 nM of dSQ12S and SQ8S, whereas commercial dye DiD failed to stain plasma membrane even at 20 nM concentration (Fig. 4).8 To the best of our knowledge, SQ8S and dSQ12S, are respectively the brightest molecular ER and plasma membrane fluorescent probes developed to date. 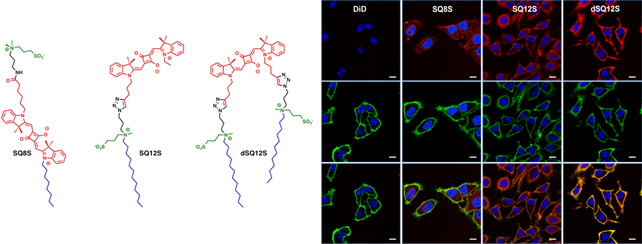 Fig. 7. (Left) Chemical structures of squaraine probes. (Right) Laser scanning confocal microscopy images of HeLa cells incubated for 5-10 minutes with the DID or squaraine probes at 20 nM without washing (red). Nucleus was stained by Hoechst (2 µg.mL-1) (blue) and the plasma membrane was stained with WGA-488 (5 µg.mL-1) (green). Scale bar is 10 µm. Adapted from.8 References
Turn-on probes for membrane receptors The classical fluorescence-based approaches to monitor ligand-receptor interactions are generally hampered by the background signal of the unbound ligand that must be removed by tedious washing steps. In collaboration with D. Bonnet and M. Hibert (UMR 7200) we initially developed fluorescent turn-on probe (NR-PEG-CBT) based on Nile Red for a G protein-coupled receptor (oxytocin) at the surface of living cells.7 Although the new probe significantly decreased the background, its brightness and photostability were limited. To address these issues, we continued collaboration with D. Bonnet and M. Hibert, following two concepts. Within the first concept, we proposed fluorogenic dye, based on a squaraine dimer, that unfolds on changing environment from aqueous to organic, and thus turns on its fluorescence.8 In aqueous media, the dimers displayed a short-wavelength band characteristic of an H-aggregate that was poorly fluorescent, whereas in organic media, they displayed a strong fluorescence, like squaraine monomer (Fig. 9). For the best dimer, which contained a PEGylated squaraine core, we obtained a very high turn-on response (organic vs. aqueous) up to 82 (Fig. 9). To apply these fluorogenic dimers for targeted imaging, we grafted them to carbetocin (Fig. 9), a ligand of the oxytocin G protein-coupled receptor. A strong receptor-specific signal was observed for all three conjugates at nanomolar concentrations. The probe derived from the core-PEGylated squaraine showed the best specificity to the target receptor together with minimal non-specific interactions (Fig. 9). The obtained dimers can be considered as the brightest polarity-sensitive fluorogenic molecules reported to date, having ~660,000 M-1cm-1 extinction coefficient and up to 40% quantum yield, whereas far-red operation region enables both in vitro and in vivo applications.8 The proposed concept can be extended to other dye families and other membrane receptors, opening the route to new ultrabright fluorogenic dyes. 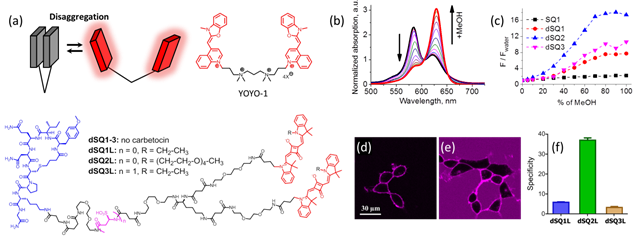 Fig. 9. Probes operating by aggregation-caused quenching mechanism. (a) Principle and examples of these probes. (b) Absorption of squaraine dimer dSQ1 in water with addition of methanol (from 0 till 100%). (c) Fluorescence intensities (F/Fwater) of dyes in H2O-MeOH mixtures with respect to their intensity in water. (d,e) Confocal images of HEK cells expressing the oxytocin receptor with 100 nM of dSQ2L (d) or rhodamine-carbetocin conjugate (e). (f) Specificity of the probes (10 nM): intensity ratio before and after addition of nonlabeled carbetocin (2 µM). Adapted from.8 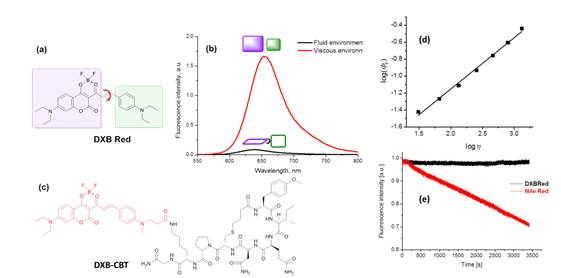 Fig. 10. Structure and rotor properties of dioxaborine molecular rotors. (a) Structure of the molecular rotor DXB Red. (b) Fluorescence response of the molecular rotor DXB Red to the change of the environment from fluid (MeOH, black) to viscous (glycerol, red). (c) Structure of the carbetocin conjugate DXB-CBT. (d) Correlation of the fluorescence quantum yield with the viscosity of the medium according to the Förster-Hoffmann equation. (e) Photostability of DXB Red and of Nile Red in toluene. Excitation wavelength was 525 nm; emission was detected at 600 nm; illumination power density was ~1 mW cm-2. Adapted from.9 In our second approach, we introduced a push-pull boron-bridged (dioxaborine) dye DXB Red that presents unique spectroscopic behavior combining solvatochromism and molecular rotor properties (Fig. 10).9 In comparison to solvatochromic and molecular rotor dyes, dioxaborine derivative shows exceptional extinction coefficient (120,000 M-1 cm-1), high fluorescence quantum yields and red/far-red operating spectral range. DXB Red also displays much higher photostability in apolar media as compared to Nile Red (Fig. 10), a fluorogenic dye of similar color. Its carboxy derivative has been successfully grafted to carbetocin (DXB-CBT, Fig. 11). This conjugate exhibits >1000-fold turn on between apolar 1,4-dioxane and water. It targets specifically the oxytocin receptor at the cell surface, which enables receptor imaging with excellent signal-to-background ratio (>130) (Fig. 11).9 We expect that presented push-pull dioxaborine dye opens a new page in the development of fluorogenic probes for bioimaging applications. 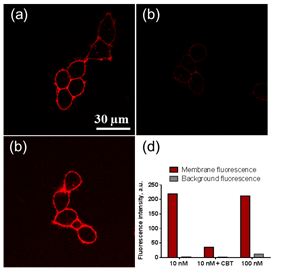 Fig. 11. Confocal images of OTR cells with 10 nM of DXB-CBT (a), 10 nM of DXB-CBT and 2 µM of CBT competitor (b), or 100 nM of DXB-CBT (c). Fluorogenic properties of DXB-CBT: average membrane and background fluorescence for all the images (d). Adapted from.9 In collaboration with a group of Michaël RYCKELYNCK (UPR9002, Strasbourg), we currently develop fluorogenic probes for detection of nucleic acids. The concept is based on “spinatch”-type assay, where initially non-fluorescent dye turns on its emission under recognition of nucleic acid aptamer. This work is under development (supported by ANR BrightRiboProbes). References
|
|||||